P426 In failure of non-optimised adalimumab (ADA) with therapeutic serum levels, a change in biotherapy class is greater than an intensification of ADA dose in IBD patients
X. Roblin1, C. genin1, N. williet1, P. veyrard1, G. boschetti2, A.E. berger3, B. flourie2, S. nancey2, S. Paul3
1Department of Gastroenterology and Hepatology- Inserm- CIC1408, CHU Saint Etienne - Hospital Nord, Saint-Priest en Jarez, France, 2Department of Gastroenterology and Hepatology, CHU Lyon sud, Lyon, France, 3CHU Saint Etienne - Hospital Nord, Immunology, Saint-Priest en Jarez, France
Background
Nearly 40% of patients who failed on ADA have therapeutic residual trough levels (pharmacodynamic failure). It is then recommended to change the therapeutic class to a non-anti-TNF biological. In this situation, however, a quarter of patients respond to an ADA dose optimisation. The purpose of this work was to compare these two strategies and identify predictors of response for each strategy.
Methods
This is a bi-centre, retrospective study based on a clinical and biological database that included patients followed from 2015 to 2016 with an IBD in maintenance treatment with ADA as monotherapy (40 mg/sc/14 days) and in loss of response (CDAI > 220 with faecal calprotectin > 250 µg/g stool for CD and Mayo score > 6 with endoscopic sub-score > 1 for UC). All patients included had residual trough ADA levels > 5 µg/ml. Relapsed patients were treated either with dose optimisation (ADA: 40 mg/sc/7 days) or other biotherapy (ustekinumab or vedolizumab, according to classic regimens and dosages). The primary endpoint was the relapse-free survival rate (defined as clinical and biological or endoscopic activity requiring therapeutic change). The survival curve analysis was performed using the Kaplan–Meier method and the log-rank test.
Results
In total, 125 patients (mean age: 38 years, 75% CD,
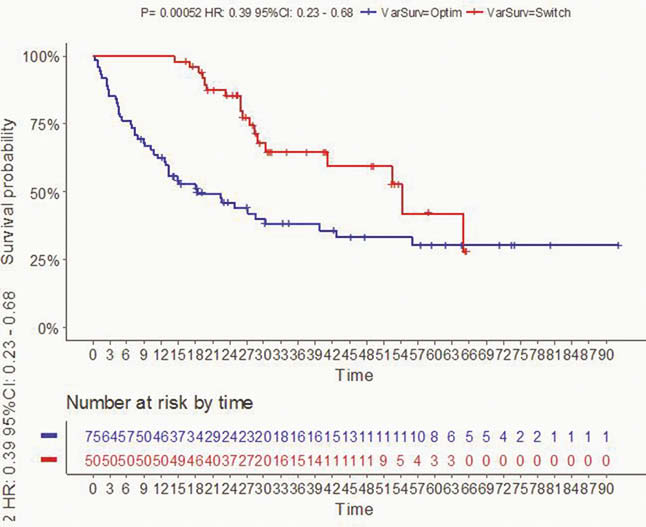
Conclusion
In patients on ADA who are losing response despite therapeutic rates, the change of class to non-anti-TNF biotherapy allows a significantly greater lasting response than a dose optimisation strategy for ADA.


