P430 Longer-term tofacitinib effectiveness for the treatment of ulcerative colitis: Two-year outcomes from a UK observational cohort study
Honap, S.(1,2);Sharma, E.(1);Ray, S.(1);Mawdsley, J.(1);Anderson, S.(1);Samaan, M.(1);Pavlidis, P.(1,2);Irving, P.(1,2);
(1)Guy’s and St Thomas’ NHS Foundation Trust, IBD Centre - Department of Gastroenterology, London, United Kingdom;(2)King's College London, School of Immunology and Microbial Sciences, London, United Kingdom;
Background
Tofacitinib is an oral small molecule inhibitor licensed for the treatment of ulcerative colitis (UC). Whilst real world data has been presented, outcomes beyond 26 weeks are limited. The objective of this study was to assess the persistence and long-term effectiveness of tofacitinib.
Methods
We conducted a retrospective observational cohort study, using a prospectively maintained database, at a large UK IBD tertiary referral centre. The primary objective was to assess the proportion of patients in deep remission at one year. Deep remission was defined as corticosteroid free clinical remission (CSFR) and endoscopic remission (Mayo Endoscopic Subscore (MES) of 0 or 1). All adult patients receiving tofacitinib for moderate to severe UC were included from October 2018 to October 2021. Disease activity was assessed using the Simple Clinical Colitis Activity Index (SCCAI); clinical response and remission were defined as a reduction of SCCAI ≥3 and a SCCAI ≤2, respectively. All analyses were based on an intention-to-treat principle, and we used non-responder imputation in cases of missingness.
Results
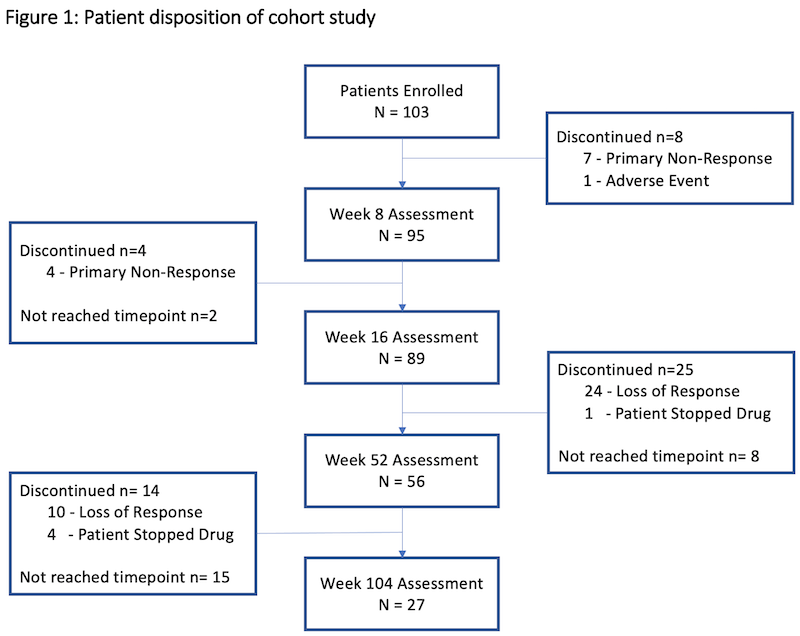
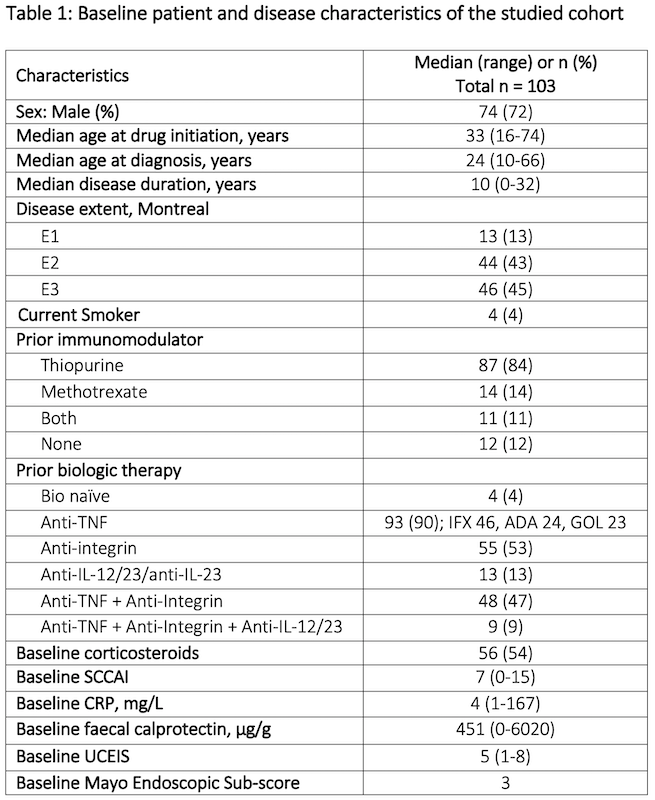
In total, 103 patients were included with a median follow up duration of 53 weeks (range 1-156). Prior to treatment, patients had active disease clinically (median SCCAI 7 (range 0-15) and endoscopically (median MES 3) with 54% on corticosteroids. The cohort had refractory disease; 90% were anti-TNF-experienced and 48% had prior exposure to at least two different biologic classes. Table 1 shows the baseline characteristics of the cohort and figure 1 shows patient disposition in the study. Median treatment duration on 10mg bd was 18 weeks (range 1-135); 27% (n=15) were on 10mg BD at one year and 11% (n=3) at two years. At 52 weeks, 61% were in CSFR, 38% in endoscopic remission, and 36% in deep remission (figure 2). Deep remission was not associated with baseline variables, including prior biologic exposure or disease severity on univariate analyses. Faecal calprotectin fell from median 451ug/g [range 5-6020] at baseline to 86ug/g [range 5-2360] at week 52 (n=41, p<0.001), but CRP change was not significant. Three-quarters of those remaining on tofacitinib at 104 weeks (n=27) were in deep remission. The Kaplan Meier estimated probability of remaining on tofacitinib was 62% (95% CI 51-71%) at week 52 and 44% (CI 95% 32-54%) at week 104 (figure 3).
Conclusion
Longer term effectiveness and treatment persistence data in a cohort of highly refractory UC patients is reassuring.