P435 Real-world clinical outcomes after elective discontinuation of first-use biologics in IBD patients
U.N. Shivaji1, A. Bazarova2, T. Critchlow3, O.M. Nardone4, S.C. Smith4, M. Love3, J. Davis3, S. Ghosh1, M. Iacucci1
1Gastroenterology, NIHR Birmingham Biomedical Research Centre- University Hospitals Birmingham NHS Foundation Trust and University of Birmingham- UK, Birmingham, UK, 2Institute for Biological Physics, University of Cologne, Cologne, Germany, 3Gastroenterology, University Hospitals Birmingham- UK, Birmingham, UK, 4Gastroenterology, Institute of Immunology and Immunotherapy- University of Birmingham, Birmingham, UK
Background
In real-world clinical practice, biologics may be discontinued due to variety of reasons, including discontinuation by gastroenterologists, in the UK. The aim of the study was to report on outcomes after discontinuation in IBD patients after a minimum follow-up of 24 months.
Methods
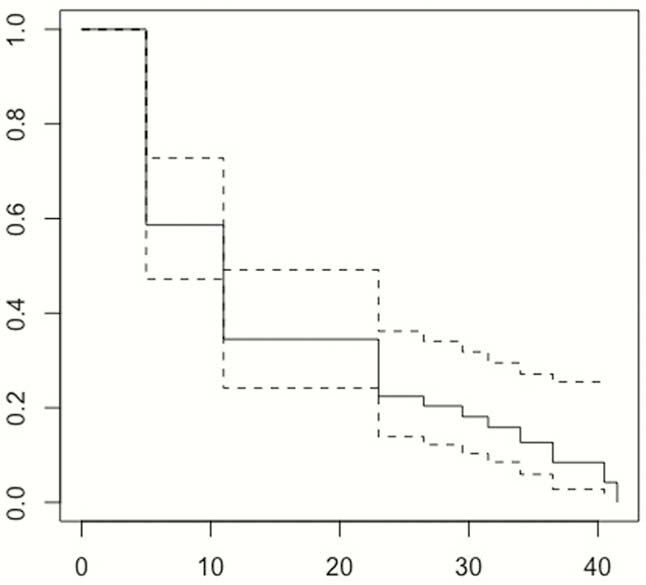
All IBD patients who discontinued their first-use biologics between January 2013 and Dec 2016 were identified from the EMR at a tertiary referral centre to ensure at least 24 months follow-up. Reasons for discontinuation and pre-defined adverse outcomes (steroid and other rescue therapies, hospitalisations, surgery including perianal) were recorded. The data were analysed using multivariable and univariable logistic regressions within a machine learning technique in order to predict adverse outcomes within the stated timeframe. We performed Kaplan-Meier survival analysis for those patients who had biologics electively discontinued.
Results
In total, 147 IBD patients who discontinued biologics (
Conclusion
The majority of patients who electively discontinued biologics had IBD-related AO rapidly after the stoppage and needed to restart biologics. Almost all patients had AO by the end of the study follow-up period. This should be discussed with patients when considering discontinuation of biologics.


