P436 Efficacy and safety of biosimilars of anti-TNF-a in paediatric-onset Inflammatory Bowel Disease: Data from the Sicilian Network for Inflammatory Bowel Diseases (SN-IBD)
Dipasquale, V.(1);Pellegrino, S.(1);Cucinotta, U.(1);Citrano, M.(2);Graziano, F.(2);Cappello, M.(3);Busacca, A.(3);Orlando, A.(4);Accomando, S.(5);Romano, C.(1);
(1)University of Messina Hospital G. Martino, Department of Human Pathology in Adulthood and Childhood "G. Barresi", Messina, Italy;(2)Pediatric Unit- Villa Sofia Cervello Hospital, Pediatric, Palermo, Italy;(3)Gastroenterology and Hepatology Section- Internal Medicine and Medical Specialties PROMISE- University of Palermo, Department of Health Promotion- Mother and Child Care, Palermo, Italy;(4)IBD Unit- Villa Sofia Cervello Hospital, Department of Medicine, Palermo, Italy;(5)G di Cristina Children's Hospital- University of Palermo, Pediatric Department, Palermo, Italy; Sicilian Network for Inflammatory Bowel Diseases (SN-IBD)
Background
Few data regarding the use of biosimilars of anti-tumour necrosis factor-a (TNF-a) in children with Inflammatory Bowel Disease (IBD) have been reported. We aimed to assess the efficacy and the safety of biosimilars of infliximab (IFX) and adalimumab (ADA) in paediatric-onset IBD.
Methods
This was a multicentre, observational, retrospective study performed among the cohort of the Sicilian Network for the Inflammatory Bowel Disease (SN-IBD) and including all patients with paediatric-onset IBD treated with the biosimilars of IFX or ADA. Demographic and clinical data were collected from medical records. PUCAI and PCDAI scores at the time of IFX start, after 14 and 54 weeks were recorded. The primary outcome was the rate of clinical remission at weeks 14 and 54. Secondary outcomes included treatment duration and incidence of adverse events.
Results
They were included 128 patients, of whom 87 on IFX biosimilar (group 1) and 41 on ADA biosimilar (group 2) (Table 1).
Table 1.Baseline characteristics of patients
At week 14, UC patients on IFX biosimilar had a median PUCAI score of 10 (IQR 5.0-22.5), while CD patients a median PCDAI score of 5 (IQR 5.00-10.8). Overall, the remission rate on IFX biosimilar at week 14 was 61.5%, and it was significantly associated with positive family history [OR 13.18 (2.44-245.65, p=0.015)], older age at starting biosimilar [OR 1.19 (1.02-1.41, p=0.038)], and diagnosis of CD (in comparison to UC) [OR 3.45 (1.35-9.23, p=0.011)]. At week 54, both UC and CD patients on IFX biosimilar had a median clinical score of 5. The remission rate was 69.1%, and no significant association with any variable was found. Patients on ADA showed a remission rate of 75% at 14 weeks, and of 70% at 54 weeks, both associated with shorter disease duration [OR 0.67 (0.45-0.93, p=0.025) and OR 0.55 (0.28-0.86, p=0.026), respectively]. At 54 weeks, patients on IFX showed a >90% failure-free survival (Figure 1), which was inversely associated with the presence of extraintestinal manifestations (HR 5.12, p=0.014) and non-naive patients (HR 3.81, p=0.025). Patients on ADA biosimilar had >83% failure-free survival at 12 months (Figure 2), associated with shorter disease duration (HR 1.41, p=0.043). About safety, a total of 13 adverse events were registered, 9 in group 1 (incidence of 6.13/100 PY) and 4 in group 2 (incidence of 12.23/100 PY). Most (n=7) were acute infusion reactions.
Figure 1.Failure-free survival on IFX biosimilar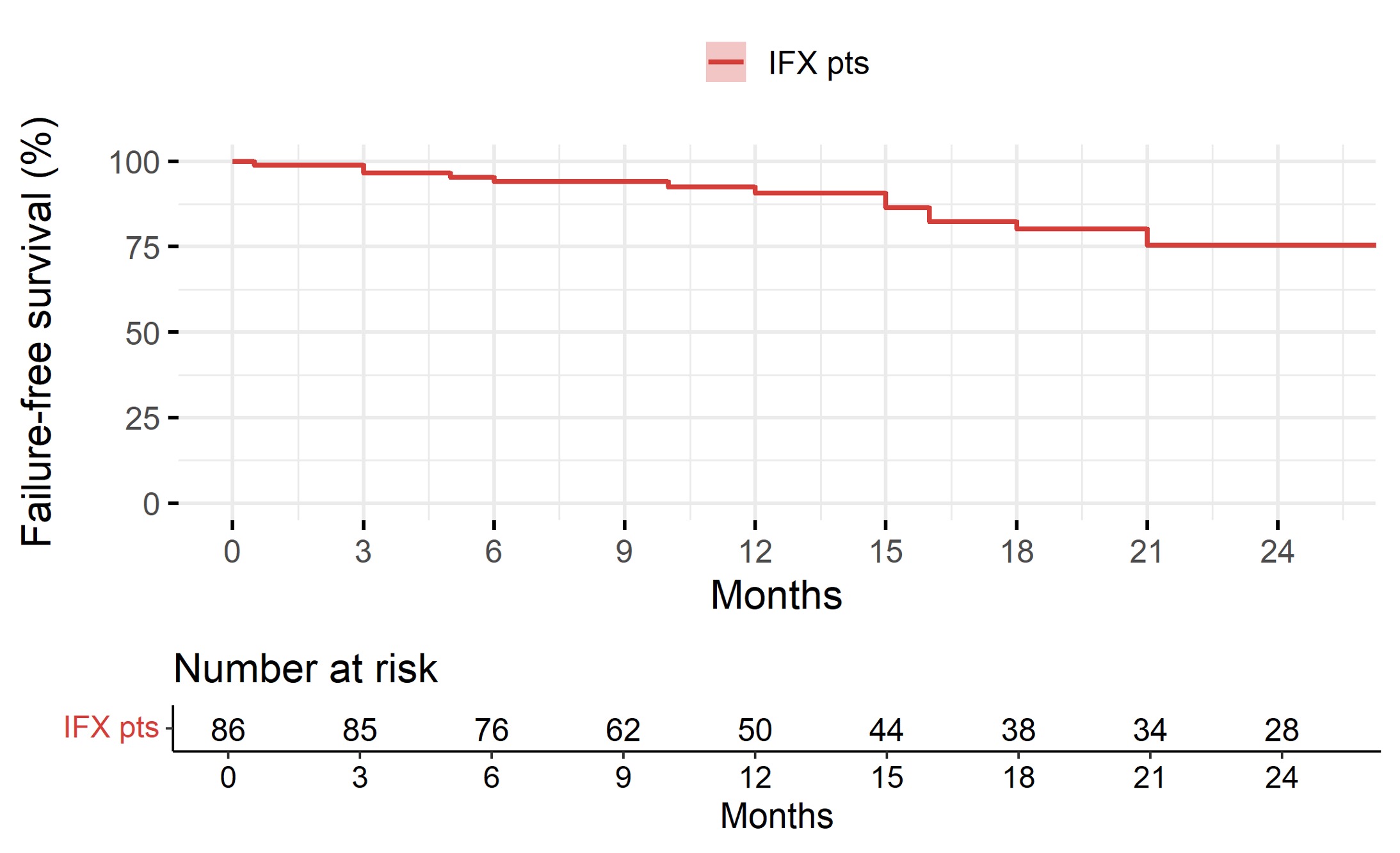
Figure 2.Failure-free survival on ADA biosimilars

Conclusion
This is one of the largest cohorts of paediatric-onset IBD on biosimilars, and confirmed these drugs are effective and safe in this group of patients, with high percentage of failure-free survival at 1 year and low incidence of mild adverse events.


