P436 Efficacy and safety of intravenous ustekinumab re-induction therapy in Crohn’s disease patients with secondary loss of response to ustekinumab maintenance therapy: Week 16 results from the POWER trial
Schreiber, S.W.(1)*;Lee, S.(2);van der Woude , C.J.(3);Marín-Jiménez , I.(4);Wolf, D.(5);Schnoy, E.(6);Salzberg , B.(7);Busse , C.(8);Nazar, M.(9);Langholff, W.(10);Borghorst, S.(11);Gasink, C.(8);Baker, T.(12);Godwin, B.(8);Feagan, B.(13);
(1)Christian-Albrechts-University and University Hospital Schleswig-Holstein, Department of Internal Medicine I, Kiel, Germany;(2)University of Washington, Division of Gastroenterology, Seattle, United States;(3)Erasmus University Medical Center, Department of Gastroenterology and Hepatology, Rotterdam, The Netherlands;(4)Hospital Universitario Gregorio Marañón- IiSGM, Gastroenterology Unit, Madrid, Spain;(5)Atlanta Gastroenterology Associates, Center for Crohn's Disease & Ulcerative Colitis, Atlanta, United States;(6)University Hospital of Augsburg, Internal Medicine III- Gastroenterology, Augsburg, Germany;(7)IBD Center of Atlanta, Atlanta Gastroenterology Specialists PC, Atlanta, United States;(8)Janssen Scientific Affairs- LLC, Gastroenterology, Horsham, United States;(9)Janssen-Cilag Polska Sp. z.o.o., Gastroenterology, Warsaw, Poland;(10)Janssen Research & Development- LLC, Biostatistics, Spring House, United States;(11)Janssen-Cilag GMBH, Health Economics/Market Access/Reimbursement, Neuss, Germany;(12)Janssen Research & Development- LLC, Immunology, Spring House, United States;(13)University of Western Ontario, Division of Gastroenterology- Department of Medicine, London, Canada; POWER Investigators
Background
After successful induction, a subset of patients with Crohn’s disease (CD) experience a secondary loss of response (LoR) to ustekinumab (UST) maintenance therapy. Dose intensification may assist in regaining response. The phase 3b randomised, double-blind, multicentre POWER study evaluated efficacy and safety of a single intravenous (IV) re-induction UST dose vs continued UST subcutaneous (SC) treatment in CD patients with LoR to standard UST every 8 weeks (q8w) maintenance therapy.
Methods
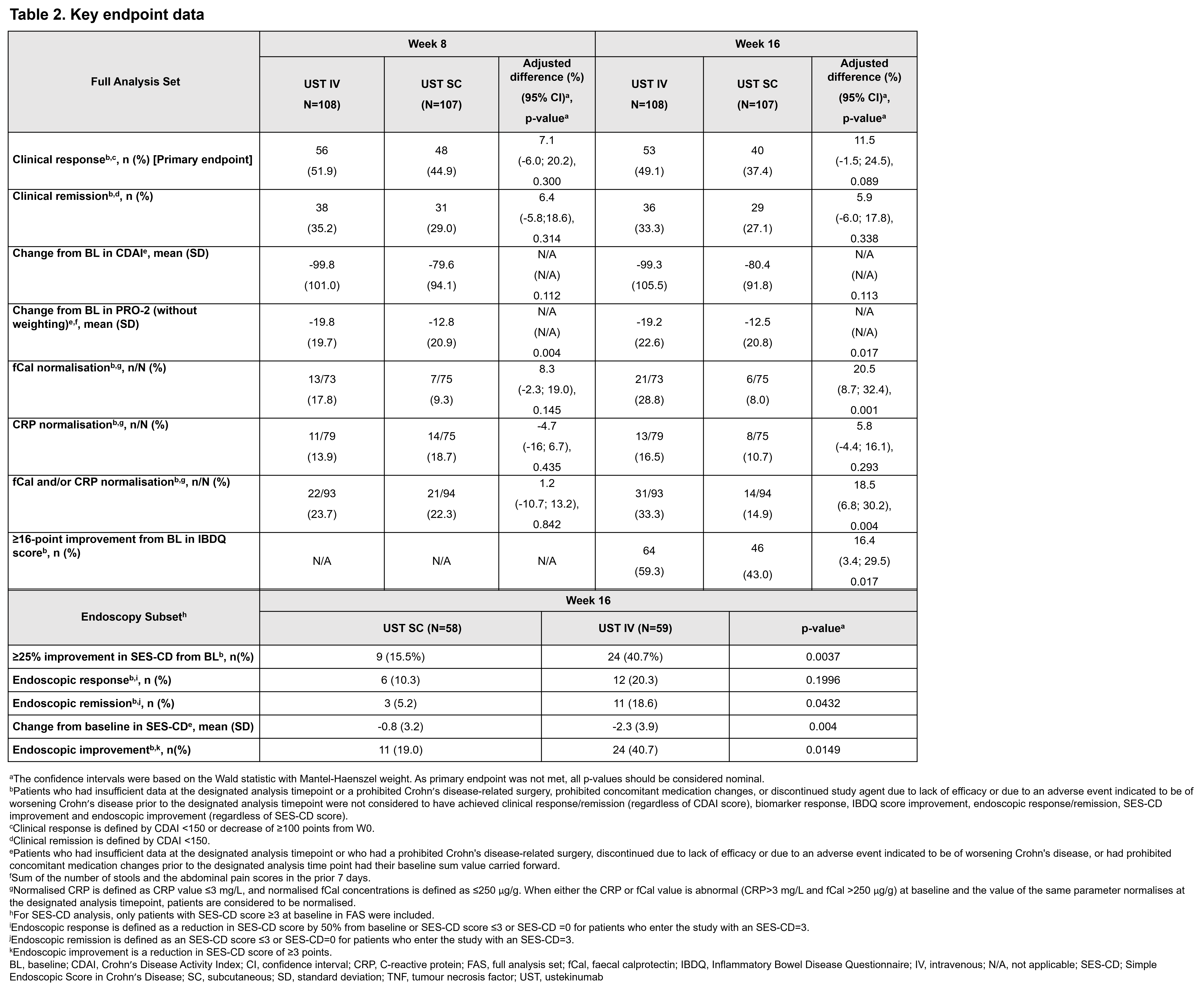
Adults with moderately–severely active CD who initially responded to UST IV induction therapy per label and subsequently experienced LoR were included. LoR for inclusion was defined as CDAI score of ≥220 and ≤450, in addition to elevated CRP (>3mg/L), fCal (>250mg/kg) or endoscopy performed ≤3 months before W0 with evidence of active CD (≥1 ulcerations in the ileum and/or colon). At baseline (W0), randomised patients received either ~6mg/kg IV UST/SC placebo (IV arm), or IV placebo/SC UST 90mg (SC arm), followed by SC UST 90mg dosing in both groups at W8/16. Clinical and biomarker assessments were made at W0/8/16 and optional ileocolonoscopy at W0/16. Primary endpoint: clinical response (CRes; decrease of ≥100 points from W0/CDAI <150) at W16. Additional outcome measures included clinical, biomarker, endoscopic and quality of life endpoints assessed at W8/16.
Results
The analysis set comprised 215 patients at W0 (IV, n=108; SC, n=107). At W16, 92.6% and 86.0% completed treatment from IV and SC arms, respectively. In both arms (IV, 58.3%; SC, 57.9%) most patients experienced ≥2 biologic failures before initiating UST (Table 1). At W16, 49.1% in the IV arm and 37.4% in the SC arm achieved CRes (p=0.089). Percentages of patients with normalisation of fCal, normalisation of CRP and/or fCal, endoscopic remission and improvement, and improvement in IBDQ score were greater in the IV vs SC arm (Table 2). At W16, similar proportions of patients had ≥1 adverse event (AE) (IV, 60.2%; SC, 61.7%) and serious AEs (IV, 5.6%; SC, 5.6%). The proportions of patients with infections were similar between arms (IV, 23.1%; SC, 21.5%), with only 1 serious infection in each arm.

Conclusion
POWER is the first randomised, controlled, double-blind trial to assess the efficacy and safety of UST IV re-induction in patients with LoR during UST maintenance therapy. The CDAI-based primary endpoint at W16 was not met. However, patients in this heavily pre-treated population who received IV re-induction showed clinically meaningful improvements at W16 compared with those receiving SC, particularly for objective endpoints, including inflammatory biomarkers and endoscopic outcomes.


