P438 Efficacy and safety of ustekinumab for Ulcerative Colitis through 4 years in Asian patients: Final results from the sub-population of UNIFI long-term extension
Hisamatsu, T.(1)*;Kim, H.J.(2);Motoya, S.(3);Suzuki, Y.(4);Ohnishi, Y.(5);Marano, C.(6);Imai, Y.(7);Saadoun, C.(8);Zhuo, J.(9);Kawamura, S.(8);
(1)Kyorin University School of Medicine, Department of Gastroenterology and Hepatology, Tokyo, Japan;(2)Kyung Hee University College of Medicine, Center for Crohn’s and Colitis- Department of Gastroenterology, Seoul, Korea- Republic Of;(3)Sapporo-Kosei General Hospital, IBD Center- Hokkaido Prefectural Welfare Federation of Agricultural Cooperative, Hokkaido, Japan;(4)Toho University Sakura Medical Center, Division of Gastroenterology and Hepatology- Department of Internal Medicine-, Sakura, Japan;(5)Shizuoka Medical Center, Division of Gastroenterology, Shizuoka, Japan;(6)Janssen Research & Development- LLC, Immunology, Spring House, United States;(7)Janssen Pharmaceuticals K.K. Japan, Immunology, Tokyo, Japan;(8)Janssen Pharmaceuticals Asia Pacific, Immunology, Singapore, Singapore;(9)Janssen China Research & Development, Biostatistics, Shanghai, China; UNIFI Investigators
Background
Ustekinumab (UST) is an interleukin-12/23 p40 antagonist approved for the treatment of moderately to severely active ulcerative colitis (UC). Here, we report the final efficacy and safety results of UST in the Asian subpopulation in the UNIFI long-term extension (LTE) through 4 years (Week [WK] 200).
Methods
Overall, 87 intravenous (IV) UST induction WK8 responders were randomised to subcutaneous (SC) maintenance therapy: 34 placebo (PBO); 24 UST 90 mg every 12 weeks (q12w); 29 UST 90 mg q8w. The nonrandomised patients (pts) included 22 IV UST induction nonresponders who received SC UST at WK8, responded at WK16, and continued on SC UST q8w; and 12 responders to IV PBO induction who received SC PBO.1,2 Pts who completed WK44 continued on SC UST q8w or PBO treatment in the LTE, and PBO pts were discontinued after unblinding. Starting at WK56, randomised pts with UC worsening could adjust to SC UST 90 mg q8w. Efficacy was evaluated in UST-randomised pts (n=53) using symptomatic remission (Mayo stool frequency subscore of 0 or 1 and rectal bleeding subscore of 0). Safety was evaluated for all pts treated in the LTE, including randomised and nonrandomised populations until WK220.
Results
Among all UST-randomised pts at maintenance baseline WK0 (intent-to-treat population with nonresponder imputation for missing data and treatment failure criteria), 49.1% were in symptomatic remission at WK200 (biologic naïve 77.8.%; failures 34.4%); 49.1% of pts were in corticosteroid-free symptomatic remission at WK200 (Table 1; Fig. 1-2). Overall, 43.5% of biologic failure and 0% of biologic naïve pts who were randomised to UST and treated in the LTE discontinued treatment between WK44 and WK200. Among randomised pts who continued UST in the LTE, 63.4% were in symptomatic remission at WK200; 78.6% of pts with observed data at WK200 were in symptomatic remission (Table 1; Fig. 3); and 80.0% of those in clinical remission at WK44 were in symptomatic remission at WK200. Safety events were similar among UST-treated pts compared with PBO. From maintenance baseline WK0 through WK220, pts receiving UST (combined UST) and PBO had 241.8 and 56.5 total pt-years of follow-up, respectively. Safety events per 100 pt-years of follow-up for combined UST vs PBO were AEs: 222.48 vs 320.29, SAEs: 11.58 vs 7.08, and serious infections: 1.65 vs 1.77. During the final year of the LTE, no deaths or major cardiovascular events were reported in UST-treated Asian pts.
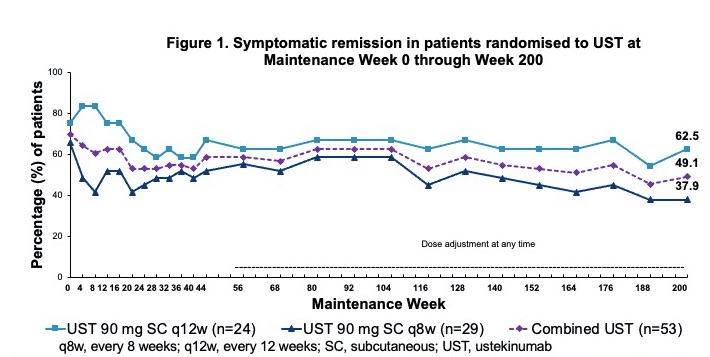
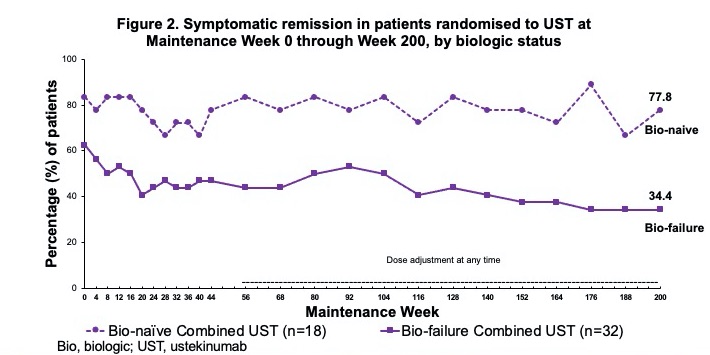

Conclusion
Consistent with the overall study population in UNIFI LTE1,2, Asian pts receiving SC UST generally maintained clinical benefit through 4 years. No new safety signals were observed.
1Afif W, et al. UEGW. Vienna, Austria, October 8-11, 2022. 2Sands BE, et al. ACG. Charlotte, NC, October 21-26, 2022


