P441 Adalimumab versus ustekinumab as first-line biological in a real-life cohort of moderate-to-severe Crohn's disease.
Moens, A.(1);Verstockt, B.(1);Alsoud, D.(2);Sabino, J.(1);Ferrante, M.(1);Vermeire, S.(1);
(1)University Hospitals Leuven, Department of gastroenterology and Hepatology, Leuven, Belgium;(2)Catholic University Leuven, Department of chronic diseases- metabolism and aging, Leuven, Belgium;
Background
As therapeutic options in Crohn’s disease (CD) are growing, new challenges including choice of first line therapy arise. Both adalimumab (ADM) and ustekinumab (UST) are effective at inducing and maintaining endoscopic remission in moderate-to-severe CD. Recently, the SEAVUE trial (Sands et al. DDW 2021) did not show a difference in (steroid-free) clinical remission nor endoscopic remission at week 52 in biological-naive patients with moderate-to-severe CD. Our aim was to explore if these results withstand in a real world setting of biological-naive CD patients starting their first biological.
Methods
This retrospective cohort study included adult, biological-naive CD patients starting ADM or UST between 2017-2020 in our tertiary referral centre. Patients were eligible for biological therapy as per reimbursement criteria and all had endoscopy-proven moderate-to-severe disease (at least one ulcer of any size) prior to start of therapy. ADM could be dose optimized. Clinical remission was defined as a Harvey-Bradshaw Index (HBI) < 5. Endoscopic remission (SES-CD<3) and improvement (≥50% reduction in SES-CD) were assessed at W26-52. Treatment persistence was defined as still being on ADM or UST by the end of follow-up. Missing values were imputed as nonresponses for binary outcomes.
Results
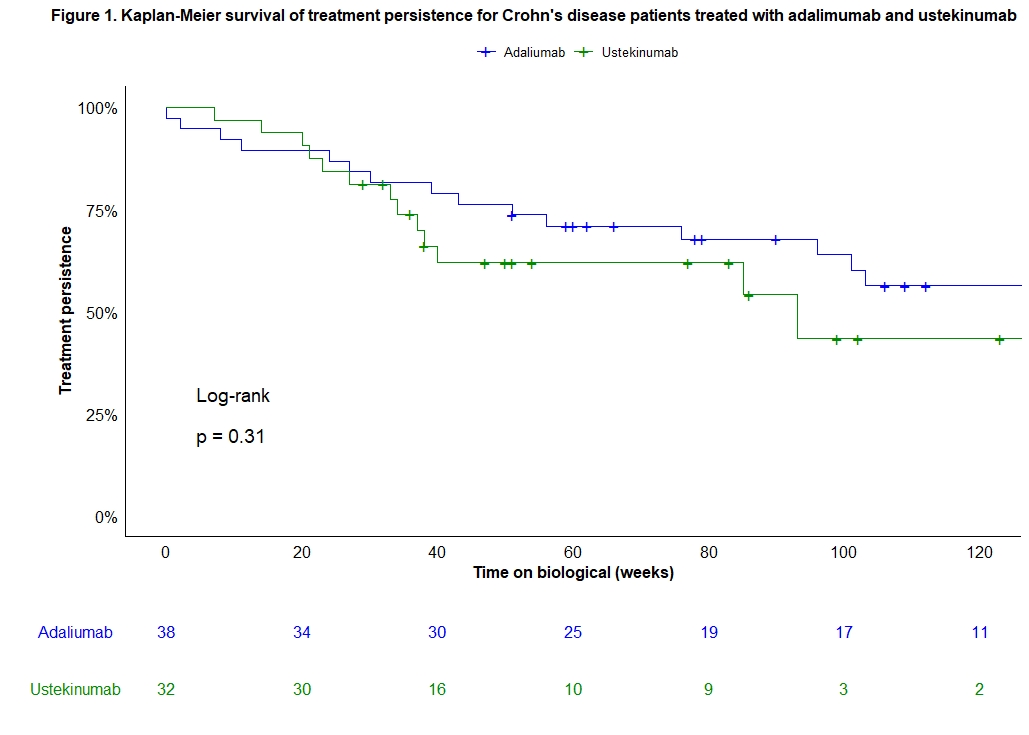
A total of 70 consecutive biological-naive CD patients were included (38 ADM, 32 UST) and prospectively followed for 60 (33-104) weeks. Baseline characteristics were similar between both treatment groups (Table 1). Median (IQR) time to endoscopy was comparable between both groups [ADM 26 (23-33) vs. UST 25 (24-45) weeks; p=0.38]. Clinical remission rates, only in patients with active clinical disease at baseline, at week 26 (ADM: 72% vs. UST: 48%, p=0.09) and 52 (ADM: 62% vs. UST: 35%, p=0.09) were numerically but not statistically different between groups. However, ADM was superior to UST in achieving endoscopic remission (61% vs. 31%, p=0.02) and improvement (74% vs. 47%, p=0.03) at week 26-52. Fifteen (39%) patients in the ADM and 6 (19%) in the UST group were on steroids at baseline. Numerical differences in steroid-free endoscopic remission (55% vs. 31%, p=0.06) and improvement (68% vs. 47%, p=0.09) rates were seen between ADM and UST at week 26-52. Rates of treatment persistence were not significantly different between both treatment groups (p=0.31; Figure 1). The number of adverse events was similar in both groups after one year of therapy (p=0.98).
Conclusion
In a real-world cohort of biological-naive CD patients, ADM seems superior to UST in achieving endoscopic remission and improvement, but no difference was seen in clinical remission at W26-52.


