P444 A systematic literature review (SLR) of real-world studies of advanced therapies in moderate-to-severe ulcerative colitis (UC): study, patient and clinical characteristics, and outcomes assessed
Irving, P.M.(1);Hur, P.(2)*;Gautam, R.(3);Guo, X.(4);Vermeire, S.(5);
(1)Guy's and St Thomas' Hospital- Guy's and St Thomas' NHS Foundation Trust, Gastroenterology, London, United Kingdom;(2)Pfizer Inc, HEOR - Inflammation and Immunology, New York, United States;(3)EVERSANA Pvt. Ltd., HEOR/Real-World Evidence, Mumbai, India;(4)Pfizer Inc, Gastroenterology- Inflammation and Immunology, Collegeville- PA, United States;(5)University Hospitals Leuven, Department of Gastroenterology & Hepatology, Leuven, Belgium;
Background
Variability in real-world (RW) study designs is often seen as a limitation for drawing conclusions. With more RW studies assessing effectiveness and safety of advanced therapies (ATs) in patients (pts) with moderate to severe UC, there is a need to better understand the study characteristics, clinical characteristics, treatments (Tx), and outcomes.
Methods
We conducted a SLR as per methodology described by Cochrane Collaboration and PRISMA guidelines, using Embase®, MEDLINE®, and MEDLINE®-In Process databases to find RW studies assessing biologics [bio] (adalimumab, golimumab, infliximab, vedolizumab/VEDO, and ustekinumab) or small molecules (filgotinib/FIL, ozanimod/OZA, and tofacitinib/TOFA) for adults with UC. Only products approved at the time of search were included. Studies published in English as full papers (Jan-2005–Feb-2022)or conference abstracts (Jan-2019–Feb-2022) were included. Studies with <30 pts or with only bio-naïve pts were excluded.
Results
From 3930 search results, 139 studies were included. Of these, 75% were published between 2019–2022; 64% were retrospective observational; and 53% were from 5 countries (Italy, US, Spain, UK, and Belgium). The median age of pts across studies was 26–55 years and male proportion ranged 25%–73% (Fig 1). 13% of studies had only bio-exposed pts, 63% had >50% pts bio-exposed and 28% presented sub-analysis for bio-exposed pts.
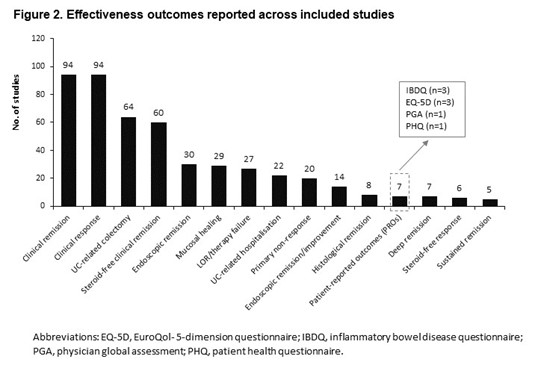
Most studies (87%) were single arm; VEDO (41%) was the most evaluated Tx, followed by TOFA (20%). No studies were found for FIL or OZA. Clinical remission (CR; 69%), clinical response (69%), and steroid-free CR (46%) were commonly reported outcomes, whereas histologic (6%) and sustained remission (4%), and patient-reported outcomes (PROs; 5%) were uncommon (Fig 2). In studies reporting CR, Partial Mayo Score (PMS) or Full Mayo Score ≤2 alone or combined with rectal bleeding and stool frequency subscore ≤1 were commonly used definitions, followed by PMS ≤1 and Simple Colitis Clinical Activity Index ≤2. Most studies assessed outcomes within 52 weeks (76%). Any adverse event (AE; 46%), colectomy (46%), withdrawal due to AE (37%), and infections (24%) were frequently reported safety events. Only up to 12% of studies reported data on MACE, malignancies and VTE.
Conclusion
More than half of RW studies in moderate to severe UC originated from five countries with VEDO and TOFA being the most frequently assessed ATs. Comparative studies were limited. Several definitions of CR were used to describe effectiveness, and few studies assessed PROs and effectiveness beyond 1 year. Inconsistent reporting of safety events was identified. Future research should consider these findings when designing a study.


