P445 Does The Sequence Matter ? Comparative effectiveness of a second line biologic in patients with Ulcerative Colitis: vedolizumab followed by an anti-TNF versus anti-TNF followed by vedolizumab
Miller, C.(1);Kwok, H.(1);Parisi, I.(1);Harrow, P.(1);McCartney, S.(1);Vega, R.(1);Rahman, F.(1);Seward, E.(1);Mehta, S.(1);Lim, S.(2);Sharma, E.(2);Samaan, M.(2);Bancil, A.(3);Kok, K.(3);Shalabi, A.(4);Johnston, E.(4);Katarey, D.(5);Taherzadeh, N.(5);Murray, C.(5);Sharip, M.(6);Carter, M.(6);Radhakrishnan, S.(7);Peake, S.(7);Khakoo, I.(8);Wahed, M.(8);Povlsen, S.(9);Patel, M.(9);Dubois, P.(9);Finkel, J.(10);Onnie, C.(10);Bloom, S.(1);
(1)University College Hospital, Gastroenterology Department, London, United Kingdom;(2)Guy's and Thomas' Hospital, Gastroenterology Department, London, United Kingdom;(3)Royal London Hospital, Gastroenterology Department, London, United Kingdom;(4)West Middlesex Hospital, Gastroenterology Department, London, United Kingdom;(5)Royal Free Hospital, Gastroenterology Department, London, United Kingdom;(6)Lister Hospital, Gastroenterology Department, Stevenage, United Kingdom;(7)St Mary's Hospital, Gastroenterology Department, London, United Kingdom;(8)Chelsea and Westminster Hospital, Gastroenterology Department, London, United Kingdom;(9)King's College Hospital, Gastroenterology Department, London, United Kingdom;(10)Whittington Hospital, Gastroenterology Department, London, United Kingdom
Background
Drug choice and order in Inflammatory Bowel Disease (IBD) is an important challenge and is becoming increasingly complex. There are few studies comparing head-to-head outcomes in second line treatments in Ulcerative Colitis (UC). It is unclear if using anti-Tumour Necrosis Factor-a (anti-TNF) therapy following vedolizumab (VDZ) or VDZ after anti-TNF has a more favourable outcome in UC in a real-world outpatient setting.
Methods
Patients with UC who were exposed to first-line anti-TNF (adalimumab/ADA or infliximab/IFX) or VDZ who subsequently switched to the alternate class between May 2013-August 2020 were identified following a review of databases at 10 hospitals. 88 VDZ and 39 anti-TNF (12 ADA,27 IFX) second line patients were eligible. Data was collected retrospectively. Baseline demographics, disease activity indices, colectomy rates, treatment persistence and healthcare resource utilisation composite endpoint (HRUC) were examined over a 52 week period for the second line biologic. HRUC included unplanned emergency hospital attendance or hospital admission.
The primary endpoints of 52 week treatment persistence, HRUC survival and colectomy free survival were analysed with Kaplan Meier method, statistical significance between the survival curves was assessed with Log Rank test. Propensity score matching (PSM) was applied to survival curves (tolerance level 0.1). For a subset where SCCAI scores available, week 52 corticosteroid free clinical response/remission rates were calculated (response: reduction of SCCAI ≥3 and remission: SCCAI ≤2).
Results
The second line anti-TNF group had a significantly higher baseline endoscopic Mayo score (p=0.035) and lower concomitant immunomodulator use (p=0.001). Second line week 52 treatment persistence was higher in the VDZ group 71/80 (89%) vs. Anti-TNF 15/36 (42%) ,p<0.0001 (Figure 1). Second line week 52 HRUC survival was higher in the VDZ group 68/81 (84%) vs. anti-TNF 20/33 (61%), p=0.003 (Figure 2). Week 52 colectomy free survival VDZ 77/80 (96%) vs. anti-TNF 26/32 (81%), p= <0.011 (Figure 3). For treatment persistence and colectomy free survival statistical significance was maintained with PSM. Week 52 corticosteroid free clinical remission rates VDZ 22/32 (69%) vs. anti-TNF 5/19 (25%) p=0.004 (Figure 4).

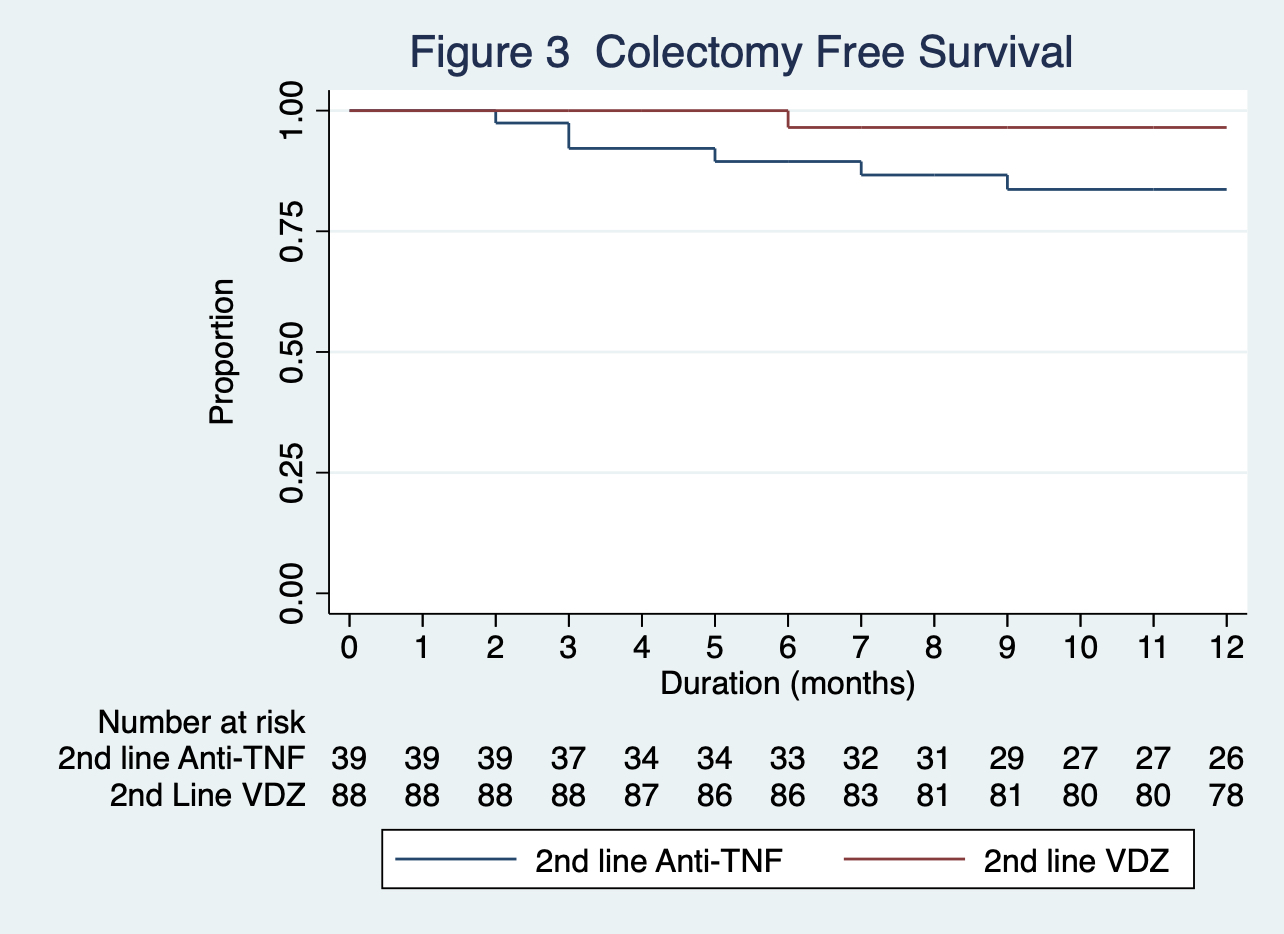
Figure 4
Conclusion
The VDZ second line cohort had significantly higher 52-week treatment persistence, lower HRUC and lower colectomy rates and higher corticosteroid free clinical remission rates. This data suggests that VDZ is an effective biologic in UC in a second line therapy after anti-TNF exposure. It highlights the effect of biologic sequencing on clinically important outcomes in an outpatient setting. Larger prospective studies are required to confirm these findings.


