P445 Network meta-analysis to evaluate the comparative efficacy of intravenous and subcutaneous infliximab and vedolizumab in the maintenance treatment of adult patients with Crohn’s Disease and Ulcerative Colitis
Peyrin-Biroulet, L.(1);Bossuyt, P.(2);Bettenworth, D.(3);Loftus Jr., E.V.(4);Anjie, S.(5);D’Haens, G.(5);Saruta, M.(6);Arkkila, P.(7);Kim, D.H.(8);Choi, D.(8);Reinisch, W.(9)*;
(1)Centre Hospitalier Régional Universitaire de Nancy, Department of Gastroenterology, Nancy, France;(2)Imelda GI Clinical Research Centre, Imelda General Hospital, Bonheiden, Belgium;(3)University of Münster, Medical Faculty, Münster, Germany;(4)Mayo Clinic College of Medicine and Science, Division of Gastroenterology and Hepatology, Rochester, United States;(5)Amsterdam UMC University of Amsterdam, Department of Gastroenterology and Hepatology, Amsterdam, The Netherlands;(6)The Jikei University School of Medicine, Department of Internal Medicine, Tokyo, Japan;(7)Helsinki University Hospital, Department of Gastroenterology, Helsinki, Finland;(8)Celltrion Healthcare Co., Ltd, Incheon, Korea- Republic Of;(9)Medical University of Vienna, Department of Internal Medicine III, Vienna, Austria;
Background
Network meta-analysis (NMA) using randomised controlled trial (RCT) data can provide indirect evidence on comparative efficacy of various treatments.1 The NMA reported herein was conducted to evaluate infliximab (IFX) and vedolizumab (VDZ) comparative efficacy during maintenance treatment of moderate-to-severe Crohn’s disease (CD) and ulcerative colitis (UC), covering various dosing regimens and administration routes for each biologic.
Methods
Studies were identified by literature searches that included publications up to 1 November 2022. Parallel-group RCTs evaluating IFX or VDZ (intravenous [IV] or subcutaneous [SC]) for maintenance treatment of adult patients with moderate-to-severe CD or UC that reported clinical remission rates were included. Eligible studies treated patients for a minimum of 22 weeks, with follow-up of 30–60 weeks for maintenance. Clinical remission rates in tumour necrosis factor inhibitor (TNFi)-naïve patients from each study were analysed in a Bayesian NMA fixed-effect model.
Results
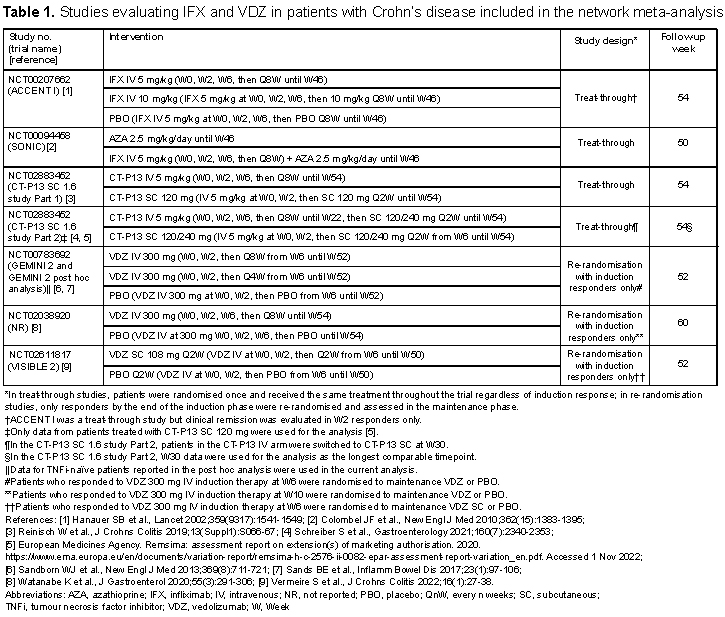
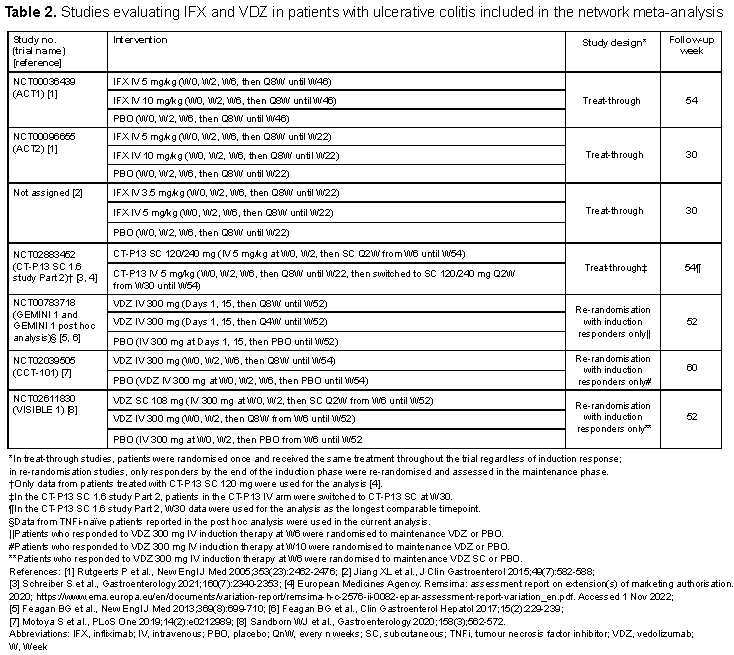
Overall, 13 RCTs were identified and included in the analysis (Table 1 for CD and Table 2 for UC). The difference in study design between IFX (treat-through) and VDZ (re-randomisation of induction responders only) was noted. A connected network of evidence could be generated using CD and UC studies (Figures 1A and 1B, respectively). In both CD and UC, IFX SC 120 mg had the highest odds ratio (95% confidence interval [CI]) vs. placebo for clinical remission during the maintenance phase (CD: 5.90 [1.90–18.2]; UC: 5.45 [1.94–15.3]), albeit with the CIs overlapping with the CIs of the other tested regimens (Figures 2A and 2B). In both CD and UC, IFX SC 120 mg ranked highest for clinical remission among the biological agents, dosing regimens, and routes of administration tested.
Conclusion
In both CD and UC, IFX SC showed a favourable efficacy profile for achieving clinical remission during maintenance treatment of TNFi-naïve adult patients, when compared with the other IFX IV or VDZ IV/SC regimens tested.
References
1 Rouse B et al., Intern Emerg Med 2017;12(1):103-111.