P450 Real life Effectiveness and safety of an induction treatment with tofacitinib in patients with Ulcerative Colitis: First results of the French TOFAST cohort
Fumery, M.(1)*;Bouhnik, Y.(2);Faure, P.(3);Bouzidi, A.(4);Brault, Y.(5);Peyrin-Biroulet, L.(6);Laharie, D.(7);
(1)CHU Amiens, Gastroenterology and Hepatology, Amiens, France;(2)Groupe hospitalier Ambroise Paré - Hartmann, Institut des MICI, Neuilly sur Seine, France;(3)Clinique Pasteur, Hepatology and gastroenterology, Tououse, France;(4)Pfizer, Infammation & Immunology, Paris, France;(5)Pfizer, Inflammation & Immunology, Paris, France;(6)University Hospital, Gastroenterology, Nancy, France;(7)Hepatology and Gastroenterology, CHU Bordeaux, Bordeaux, France;
Background
We report the results of TOFAST, a French ongoing prospective observational multicenter study, evaluating the clinical benefit of tofacitinib for the treatment of moderate to severe UC.
Methods
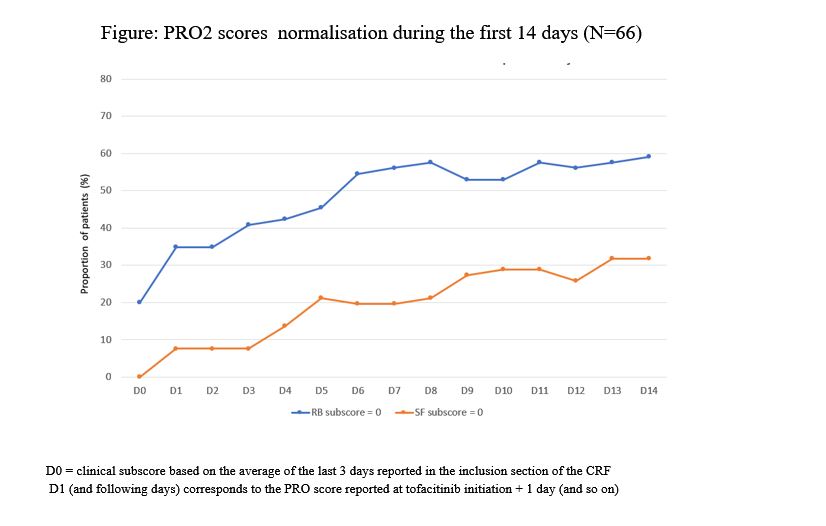
In this descriptive interim analysis, effectiveness and safety were assessed at the end of induction therapy (up to 4 months after tofacitinib initiation). Clinical response was defined as a decrease in partial Mayo score (PMS) (≥ 3 points and ≥ 30%) from inclusion with a concomitant decrease in rectal bleeding (RB) subscore ≥ 1 point (absolute subscore of 0 or 1). Clinical remission was defined as PMS ≤2 with no subscore >1. Change in daily PRO2 scores [RB and stool frequency (SF)] during the first 14 days and the median time to patient relief were also analyzed.
Results
Among the 102 patients included, 86 had available data at the first follow-up visit, corresponding to the end of induction period. At inclusion, 53.9% of patients were male with a mean (± SD) age of 37.1 ±13.6 years, a median disease duration of 6.1 years [Q1; Q3: 2.8; 10.8]. 48% of patients had pancolitis and 24.5% an extraintestinal manifestation; mean PMS was 6 ±1.8 with median SF and RB scores of 3.0 [Q1; Q3: 2.0; 3.0] and 2.0 [1.0; 2.0], respectively. Regarding prior drug exposure, 92.2% of patients had received at least one anti-TNF agent, 62.7% vedolizumab and 34.3% ustekinumab. Tofacitinib was initiated at 10 mg b.i.d, in combination with 5-ASA and corticosteroids for 19.6% and 33.3% of patients, respectively. At the first follow-up visit (median 1.9 months after treatment initiation), tofacitinib was ongoing in 87.2%, mostly (73.3%) at 10 mg b.i.d. Corticosteroids were ongoing in 29.1% of patients.
At the end of induction period, rates of clinical response and remission were 52.3% and 43%, respectively. Median changes in RB and SF scores were identical at days 7 and 14, -1 point [Q1; Q3: -1.0; 0.0] and -1 point [Q1; Q3: -2.0 - 0.0], respectively. For patients who reported improvement (68.6%), the median time to relief was 7 days [Q1-Q3: 4.0-15.0].
PRO2 scores normalisation during the first 14 days is shown in the figure.
RRegarding safety, 73.5% of patients reported at least one adverse event (AE) and 12.6% reported at least one serious AE. Ten (9.8%) infections (none considered as serious) and 2 (1.9%) Herpes Zoster were reported. No major cardiovascular or venous thromboembolic event, nor malignancy or death were observed (Table).
Conclusion
The interim results of TOFAST study show that at the end of induction therapy with tofacitinib, 52.3% of patients achieved clinical response and >40% achieved remission. Rapid improvement in SF and RB scores was observed within 2 weeks. AEs were consistent with the known tofacitinib safety profile.