P457 Treatment duration of ustekinumab in patients with Crohn’s disease (CD) and psoriasis (PSO) – real-world evidence from German claims data
Kosa, F.(1);Nair, S.(1);He, J.(1);Lin, X.(1);Di Scala, L.(1);Lehne, M.(2)*;Ghiani, M.(3);Maywald, U.(4);Wilke, T.(3);
(1)Janssen, Global Services LLC, Raritan NJ, United States;(2)Cytel, Real World Advanced Analytics, Berlin, Germany;(3)IPAM - Institut für Pharmakoökonomie und Arzneimittellogistik e.V., n/a, Wismar, Germany;(4)AOK Plus, Unternehmensbereich Versorgung, Dresden, Germany;
Background
Ustekinumab is a monoclonal antibody approved for the treatment of inflammatory diseases such as Crohn’s disease (CD) or psoriasis (PSO). Real-world evidence about treatment persistence of patients treated with ustekinumab is scarce. This retrospective observational study uses German claims data to analyse average treatment duration of ustekinumab in CD and PSO patients in a real-world setting.
Methods
Patients with at least two confirmed outpatient diagnoses or one inpatient diagnosis of Crohn’s disease (ICD-10 code: K50*) between 01/01/2016 and 30/06/2020 and a subsequent incident prescription of ustekinumab (ATC code: L04AC05) between 01/01/2017 and 30/06/2020 were identified in the AOK PLUS database, a sickness fund with more than 3.4 million patients from the states of Saxony and Thuringia in the eastern part of Germany. Similarly, patients with at least two outpatient diagnoses or one inpatient diagnosis of psoriasis (ICD-10 code: L40*) between 01/01/2010 and 30/06/2020 and an incident prescription of ustekinumab between 01/01/2011 and 30/06/2020 were identified. Treatment durations were quantified by assessing the time from the first ustekinumab prescription until treatment discontinuation. Discontinuation was defined as a gap of at least 90 days between the end date of the last prescription and the date of the next prescription or switch to another biologic agent. Deaths were counted as treatment discontinuations, and patients were censored at the end of follow-up or disenrolment from the sickness fund. Stockpiling was taken into account (i.e., accumulated medications were assumed to be taken consecutively), and hospitalizations were considered as a part of treatment. Treatment duration was explored in a Kaplan-Meier analysis.
Results
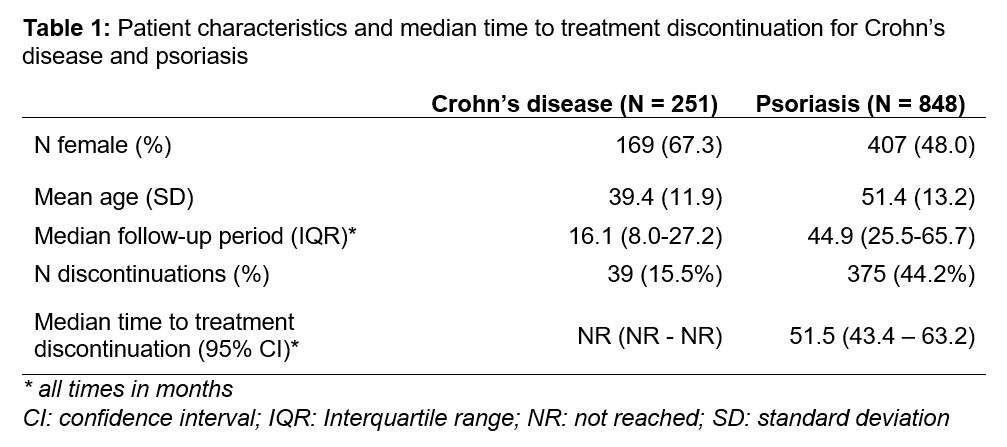
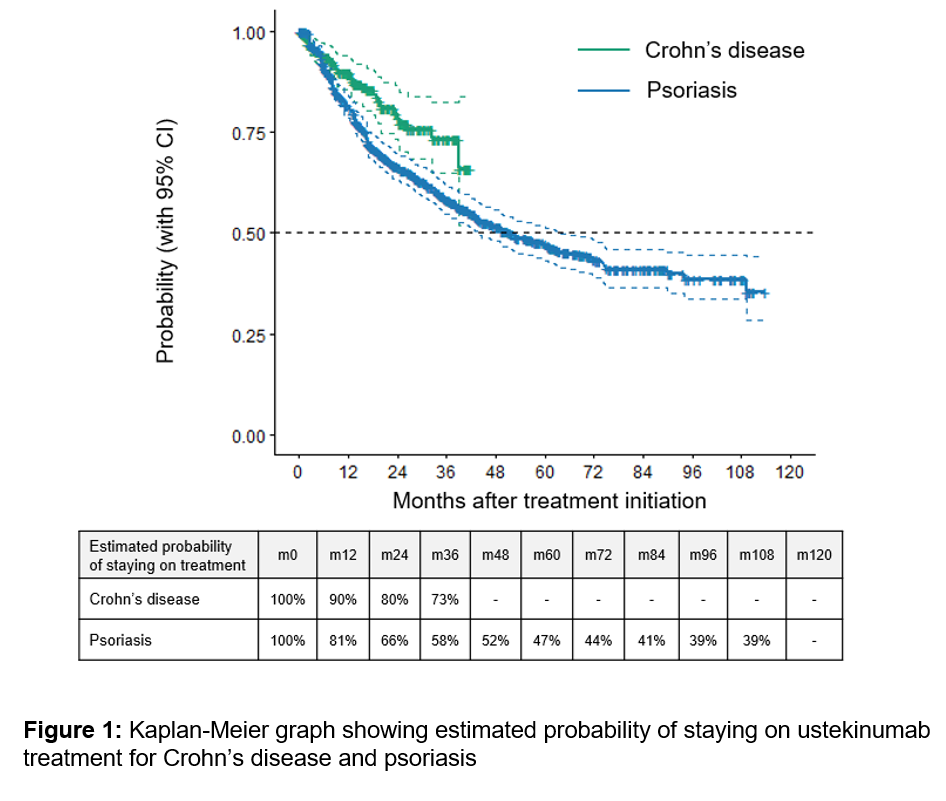
A total of 251 CD and 848 PSO patients with incident ustekinumab treatment were identified. 39 CD (15.5%) and 375 PSO patients (44.2%) discontinued treatment during the study period (median follow-up: 16.1 and 44.9 months; total patient years: 383.6 and 3338.7 for CD and PSO, respectively). For CD, median time to treatment discontinuation was not reached, as most patients were still on treatment at the end of the follow-up period; for PSO, median time to treatment discontinuation was 51.5 months (Table 1 & Figure 1).
Conclusion
Our results provide real-world evidence of ustekinumab treatment duration in patients with CD and PSO in Germany. A low proportion of CD patients discontinuing treatment over the available follow-up period and a median treatment duration of more than four years for PSO indicate that ustekinumab treatment persistence is high in these patient groups.