P462 Delayed Responders with Ustekinumab in Ulcerative Colitis Have Increased Inflammatory Burden but Similar Long-term Outcomes as Early Responders
Wong, E.(1);Dulai, P.(2);Marshall, J.(1);Jairath, V.(3);Reinisch, W.(4);Narula, N.(1)*;
(1)McMaster University, Department of Medicine Division of Gastroenterology and Farncombe Family Digestive Health Research Institute, Hamilton, Canada;(2)Northwestern University, Division of Gastroenterology, Chicago, United States;(3)Western University, Department of Medicine Division of Gastroenterology, London, Canada;(4)Medical University of Vienna, Department of Internal Medicine III Division of Gastroenterology and Hepatology, Vienna, Austria;
Background
Differences in one-year outcomes among early compared to delayed responders to vedolizumab have been demonstrated in ulcerative colitis. However, it is unclear whether differences exist with ustekinumab, and what factors differentiate delayed responders from non-responders.
Methods
This was a post-hoc analysis of patient-level data from the UNIFI clinical trial. Ustekinumab-treated patients with clinical response, defined as reduction in total Mayo score ≥30% and ≥3 points from baseline with reduction in rectal bleeding subscore (RBS)≥1 or RBS≤1, at week 8 were deemed early responders and their outcomes were compared with delayed responders (week 8 non-responders who subsequently responded at week 16). The primary outcome assessed was one-year clinical remission (CR), defined as total Mayo score ≤2 and no subscore >1. Other one-year clinical, endoscopic, and histologic outcomes were also assessed. Outcomes were compared using t-tests and biomarkers compared using one-way repeated measures analysis of variance.
Results
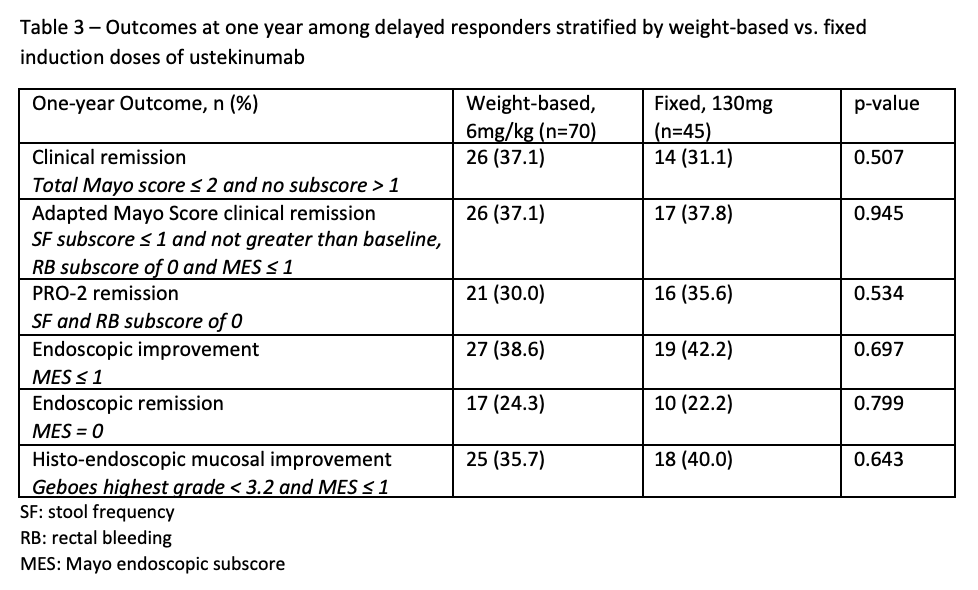
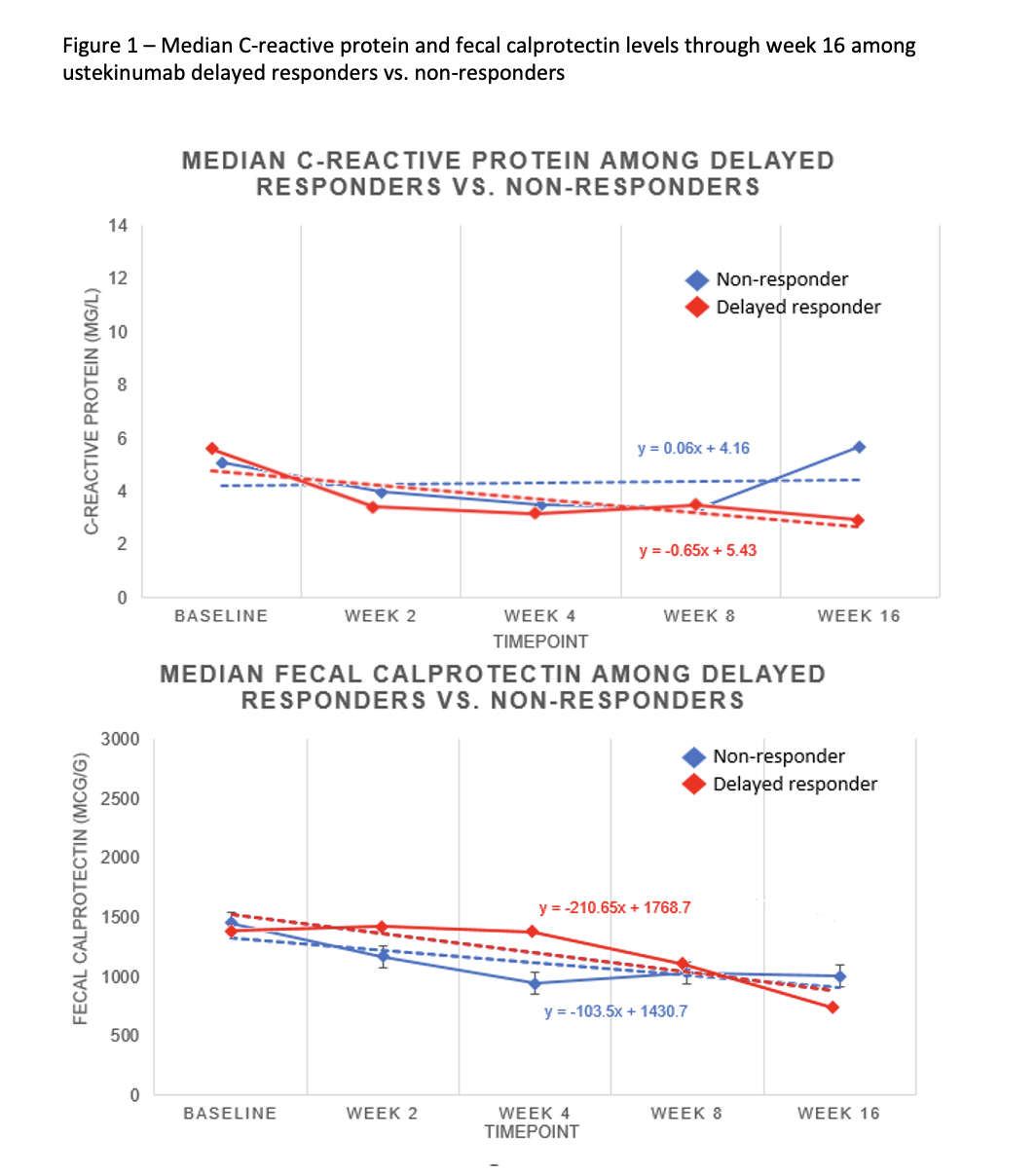
We included 642 ustekinumab-treated patients, including 321 (50%) early responders, 115 (17.9%) delayed responders, and 205 (32.1%) non-responders (Table 1). No differences were observed for one-year CR among early vs. delayed responders [132/321 (41.1%) vs. 40/115 (34.8%), p=0.233], nor for any other outcome assessed (Table 2). Similar findings were observed among delayed responders regardless of weight-based vs. fixed ustekinumab induction dose (Table 3). Compared to early responders, delayed responders had more severe endoscopic disease at baseline as measured by the MES [88/115 (76.5%) vs. 206/321 (64.2%), p=0.015] and were more likely to have abnormal CRP at baseline based on a threshold > 3mg/L [83/115 (72.2%) vs. 183/321 (57%), p=0.004]. Compared to non-responders, delayed responders had a significant decline in CRP (F(4, 844), p<0.0001) and fecal calprotectin (F(4, 818), p<0.0001) through week 16 (Figure 1).


Conclusion
Compared with early ustekinumab responders, delayed responders had greater inflammatory burden at baseline. Early and delayed responders have similar one-year outcomes. Biomarker decline can be observed in delayed responders and help differentiate them from non-responders. The decision to prolong ustekinumab among initial non-responders should be informed with biomarkers and weighing the overall risk to benefit.


