P466 Efficacy and safety outcomes in patients with moderate to severe ulcerative colitis stratified by ethnicity and race: a pooled analysis of data from GEMINI 1, VARSITY, and VISIBLE 1
Mukherjee , R.(1);Khan , R.M.Q.(1);Liu , J.J.(2);Ananthakrishnan , A.N.(3);Lichtenstein , G.R.(4)*;Uddin , S.(1);Young , L.(1);Boules , M.(1);Adamson , P.(2);Reeves , V.(5);
(1)Takeda Pharmaceuticals U.S.A.- Inc., Medical, Lexington, United States;(2)Morehouse School of Medicine, Division of Gastroenterology, Atlanta, United States;(3)Massachusetts General Hospital and Harvard Medical School, Division of Gastroenterology, Boston, United States;(4)University of Pennsylvania, School of Medicine, Philadelphia, United States;(5)GI Associates and Endoscopy Center, GI Alliance, Jackson, United States;
Background
Ulcerative colitis (UC) is an inflammatory bowel disease with an estimated prevalence of 181 cases per 100,000 adults in the USA. Hispanic or Latino individuals comprise 19% of the US population and 24% of individuals are races other than White; however, the efficacy and safety of advanced therapies for UC in these populations remains uncertain because of their underrepresentation in clinical trials. Underrepresentation has the potential to bias evidence towards therapies with understudied efficacy and safety in minority ethnic and racial groups. This post hoc analysis used pooled clinical trial data of advanced therapies in patients with UC stratified by ethnicity and race in order to identify differential efficacy and safety outcomes among the populations investigated.
Methods
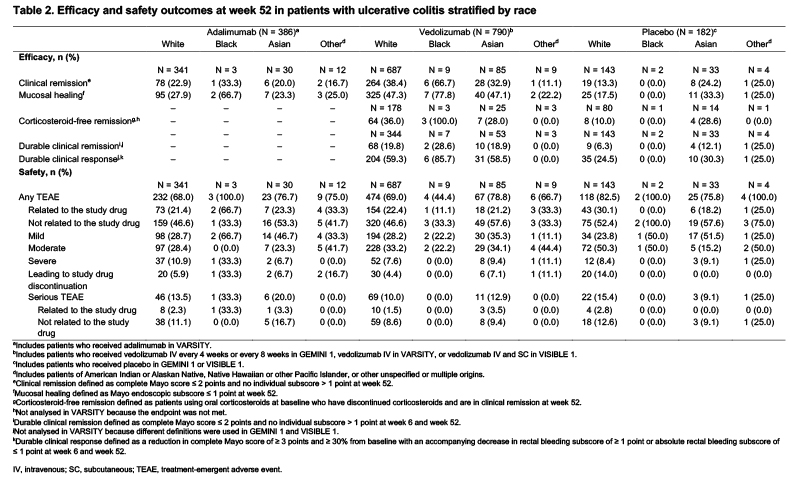
GEMINI 1, VARSITY, and VISIBLE 1 were phase 3 or 3b clinical trials investigating vedolizumab (intravenous or subcutaneous) versus placebo or adalimumab in patients with moderately to severely active UC. Data from these trials were pooled to create three cohorts of patients who received adalimumab, vedolizumab, or placebo. The number and proportion of patients with each efficacy and safety outcome at week 52 were reported for each of the treatment cohorts, stratified by ethnicity and race. Ethnicity and race were self-reported in the three trials.
Results
Overall, 1,358 patients were included in the pooled treatment cohorts. Of the 465 patients with available ethnicity information, only 40 patients (8.6%) reported their ethnicity as Hispanic or Latino. Efficacy and safety outcomes were similar when stratified by ethnicity with some exceptions (Table 1). When stratified by race, efficacy and safety outcomes were similar among White and Asian patients (Table 2). In the vedolizumab cohort, 47.3% and 47.1% of White and Asian patients, respectively, had mucosal healing. Some differences were observed in the efficacy and safety outcomes of Black patients compared with White and Asian patients; however, the treatment cohorts included fewer than 10 Black patients.
Conclusion
In the GEMINI 1, VARSITY, and VISIBLE 1 trials, efficacy and safety outcomes were similar among racial and ethnic groups, with the exception of Black patients. However, this analysis was limited by the small sample size of patients of races other than White, as well as the limited availability of ethnicity information, which prevented robust comparisons of outcomes between patients of different ethnicities and races. Future clinical trials of advanced therapies should aim to recruit and report a diverse patient population with UC who are representative of the patients who will receive these treatments.