P469 The PROPER study: interim analysis of a pan-European real-world study of SB5 adalimumab biosimilar after transition from reference adalimumab in patients with Crohn’s disease
Dignass, A.(1);Gisbert, J.(2);Freudensprung, U.(3);Addison, J.(4);
(1)Agaplesion Markus Hospital- Goethe University, Department of Medicine 1, Frankfurt, Germany;(2)Hospital Universitario de La Princesa- Instituto de Investigación Sanitaria Princesa IIS-IP- Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas CIBEREHD- Universidad Autónoma de Madrid, Gastroenterology Unit, Madrid, Spain;(3)Biogen International GmbH, None, Baar, Switzerland;(4)Biogen Idec, None, Maidenhead, United Kingdom
Background
SB5, a biosimilar to reference adalimumab, received EU marketing authorisation in August 2017, based on pre-clinical and clinical Phase I and III studies that demonstrated bioequivalence, similar efficacy, and comparable safety and immunogenicity to the reference. The ongoing real-world study ‘PROPER’ is designed to provide insights into outcomes of the transition from reference to SB5 outside the controlled, randomised, clinical trial setting, and under an umbrella design has enrolled 1,000 patients with immune-mediated inflammatory disease, treated at centres in Belgium, Germany, Ireland, Italy, Spain and the UK. The objective of this interim analysis is to describe clinical characteristics and outcomes in patients with Crohn’s disease transitioned from reference to biosimilar adalimumab SB5.
Methods
Eligible patients had been transitioned to SB5 as part of routine treatment following a minimum of 16 weeks’ treatment with reference adalimumab. Data are captured from patient charts retrospectively for 24 weeks prior to and prospectively and/or retrospectively for 48 weeks after SB5 initiation. This first interim analysis of the Crohn’s disease cohort reports outcome measures including baseline clinical characteristics, disease activity, persistence on SB5, clinical management and safety for patients enrolled at 32 specialist sites and followed up to the data extract date of February 5th, 2021.
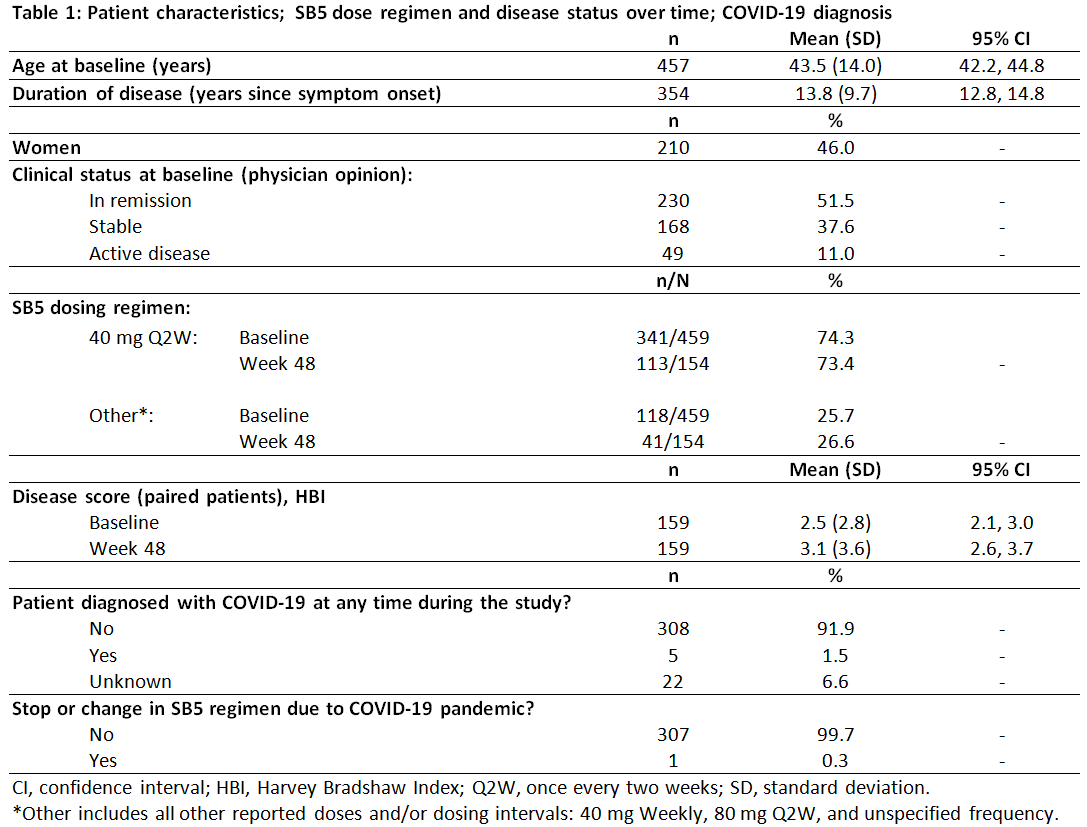
Results
Of the 459 patients included in this interim analysis, at time of data extract 108 had completed 48 weeks on SB5, 45 patients had discontinued SB5, and 10 had withdrawn from study. A disease flare was reported for 29 (6.3%) of patients, of whom 22 had no subsequent change in biologic treatment; 7 had a change, of whom 5 switched to a different biologic; 2 had secondary loss of response (physician reported). Twelve patients reported 13 serious adverse events, of which 4 (anal fistula, 2 perianal abscesses and subileus) were considered by study physician to be related to SB5 administration. In Table 1 below, baseline refers to the time of SB5 initiation.
Conclusion
This interim analysis provides a first insight into clinical management of patients in a contemporary study cohort of European patients with long-standing Crohn’s disease, transitioned from reference to biosimilar adalimumab SB5 and followed in clinical practice. The majority of patients showed no meaningful difference in disease activity or SB5 dosing regimen by Week 48 post-transition, and the Covid-19 pandemic seems to have had no impact on SB5 use in this cohort. No new safety concerns were detected.