P490 Assessment of body weight changes in patients with inflammatory bowel diseases initiating biologic therapy: A prospective cohort study
N. Borren, W. Tan, A. Jess, P.H.M. Li, J. Garber, J. Luther, F. Colizzo, H. Khalili, A. Ananthakrishnan
Division of Gastroenterology, Massachusetts General Hospital, Boston, USA
Background
Biologic therapies are effective in inducing sustained clinical and endoscopic remission in inflammatory bowel diseases. While side effects are infrequent, prior studies have inconsistently suggested that tumour necrosis factor α (anti-TNF) therapy may be associated with weight gain. We performed this prospective study to compare weight gain across different biologic therapy classes with distinct mechanisms of action.
Methods
This prospective cohort study recruited patients with moderate to severe IBD initiating outpatient biologic therapy with anti-TNF (infliximab, adalimumab), vedolizumab or ustekinumab. Weight measurements were performed at weeks 0, 14, 30 and 54. Disease activity at these time points was assessed using the Harvey Bradshaw Index (HBI) for CD and Simple Clinical Colitis Activity Index (SCCAI) for UC. Remission was defined as HBI <4 or SCCAI 2. Changes in weight between baseline and each of the follow-up visits were modelled as a continuous variable and multivariate regression assessed the independent effect of therapeutic class on this outcome.
Results
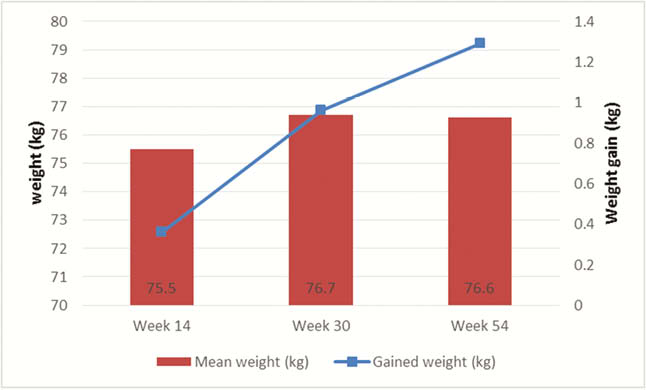
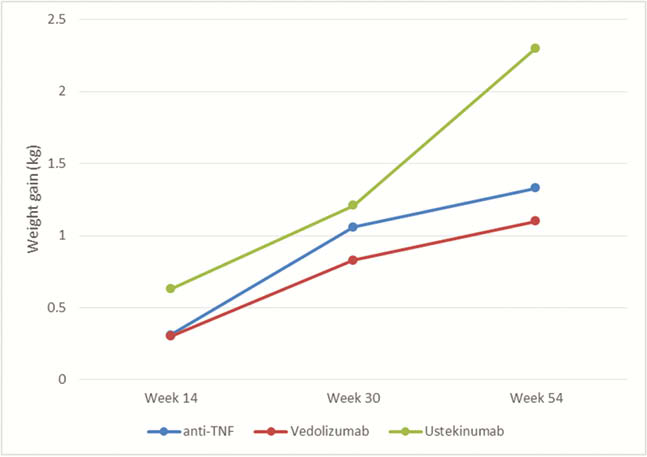
Our study enrolled 314 patients (197 CD, 117 UC) initiating biologic therapy with 120 patients starting anti-TNF (38%), 140 patients started vedolizumab (45%) and 54 patients on ustekinumab (17%). All patients provided their weight and height at baseline; 261, 184 and 131 patients provided data on weight at week 14, week 30 and week 54, respectively. The mean baseline body weight was similar among all therapeutic classes. Patients initiating UST were more likely to have Crohn’s disease (CD), have perianal involvement and have prior biologic exposure. From baseline, the weight significantly increased at week 14 with a mean of 0.36 kg ( ± 3.8kg,
Conclusion
There was no difference in weight gain between the different biologic therapeutic classes.


