P503 Early TNF-antagonist maintenance therapy results in better outcomes than immunomodulators in paediatric Crohn's disease: A multi-centre prospective cohort study
Schneider, R.(1,2);Jacobson, K.(3);Huynh, H.(4);Mack, D.(5);Deslandres, C.(6);deBruyn, J.(7);El-Matary, W.(8);Otley, A.(9);Lawrence, S.(3);Wine, E.(4);Sherlock, M.(10);Critch, J.(11);Jantchou, P.(6);Rashid, M.(9);Carroll, M.(4);Bax, K.(12);Seidman, E.(13);Benchimol, E.(1,2);Ricciuto, A.(1,2);Wu, A.(1);Pullenayegum, E.(2);Walters, T.(1);Nguyen, G.(2);Griffiths, A.(1,2);Church, P.(1,2);
(1)The Hospital for Sick Children, SickKids Inflammatory Bowel Disease Centre- Department of Paediatrics, Toronto, Canada;(2)University of Toronto, Institute of Health Policy Management and Evaluation, Toronto, Canada;(3)B.C. Children’s Hospital, Division of Gastroenterology- Hepatology and Nutrition- Department of Paediatrics, Vancouver, Canada;(4)Stollery Children’s Hospital- University of Alberta, Division of Paediatric Gastroenterology and Nutrition- Department of Pediatrics, Edmonton, Canada;(5)Children’s Hospital of Eastern Ontario- University of Ottawa, Inflammatory Bowel Disease Centre- Department of Paediatrics, Ottawa, Canada;(6)Centre hospitalier universitaire Sainte-Justine, Gastroenterology- Hepatology- and Nutrition, Montreal, Canada;(7)Alberta Children’s Hospital- University of Calgary, Pediatric Gastroenterology- Hepatology & Nutrition, Calgary, Canada;(8)Winnipeg Children’s Hospital- Max Rady College of Medicine- University of Manitoba, Paediatric Gastroenterology, Winnipeg, Canada;(9)IWK Health Centre, Division of Gastroenterology- Department of Paediatrics, Halifax, Canada;(10)McMaster Children's Hospital, Division of Gastroenterology- Department of Paediatrics, Hamilton, Canada;(11)Janeway Children’s Health and Rehabilitation Centre, Division of Gastroenterology, Newfoundland, Canada;(12)Children’s Hospital London Health Sciences Centre, Division of Gastroenterology- Hepatology- and Nutrition- Department of Paediatrics, London, Canada;(13)Montreal Children's Hospital- McGill University Health Centre, Division of Gastroenterology and Nutrition, Montreal, Canada; Canadian Children Inflammatory Bowel Disease Network
Background
Early initiation of TNF-antagonist (aTNF) therapy for Crohn’s disease (CD) is associated with greater clinical and endoscopic improvement but trialing an immunomodulator (IM) first remains common in paediatrics. This study aimed to determine if early aTNF is superior to IM maintenance therapy in paediatric luminal CD.
Methods
Children 2-17 years old with newly diagnosed CD were enrolled prospectively at one of 12 sites of the Canadian Children IBD Network. Patients with luminal inflammatory disease receiving IM or aTNF-based first maintenance therapy, following induction with steroids, exclusive enteral nutrition (EEN), or aTNF, were included. Those with perianal disease as the sole indication for aTNF were excluded. The primary outcome was sustained steroid-free clinical remission, defined by the short Pediatric CD Activity Index, at 6, 12, and 18 months, while continuing initial maintenance. A secondary outcome was combined sustained steroid-free clinical and endoscopic remission by 18 months, defined as the absence of ulcers at follow-up colonoscopy when ulcers were present at baseline. Multivariable logistic regression modeled the propensity of receiving aTNF maintenance therapy and 1:1 Greedy matching, without replacement, was used to match each of the participants who received aTNF with a participant who received an IM. The primary outcome was analyzed in the whole cohort, and in the subgroup receiving steroids or EEN as induction, using a Chi-square test, as well as in the matched group with a McNemar’s test, generalized estimating equation (GEE), and with a mixed model to account for clustering by centre. Sensitivity analyses with inverse probability treatment weights using GEE were performed.
Results
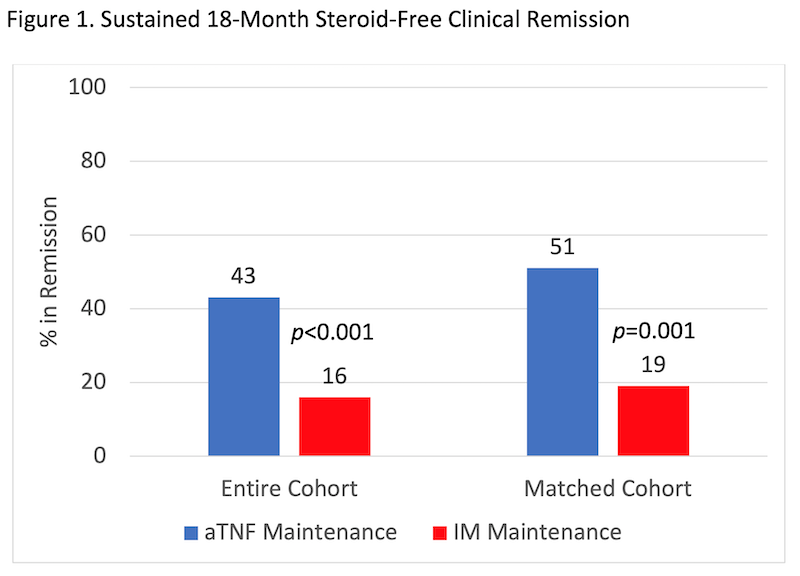
Among children with CD enrolled in the inception cohort study between 01/2014 and 12/2018, 464 met inclusion criteria and received IM (n=284) or aTNF (n=180 of whom 88 received steroids or EEN induction) as first maintenance therapy (Table 1). 162 patients were included in the matched cohort. In every analysis, more of the aTNF group achieved sustained 18-month steroid-free clinical remission, despite more severe baseline disease (Figure 1). The result remained significant in sensitivity analyses (Table 2). In patients with ulcers at baseline, 57.1% of the aTNF group versus 4.6% of the IM group achieved sustained steroid-free clinical remission and endoscopic remission by 18 months (p<0.001).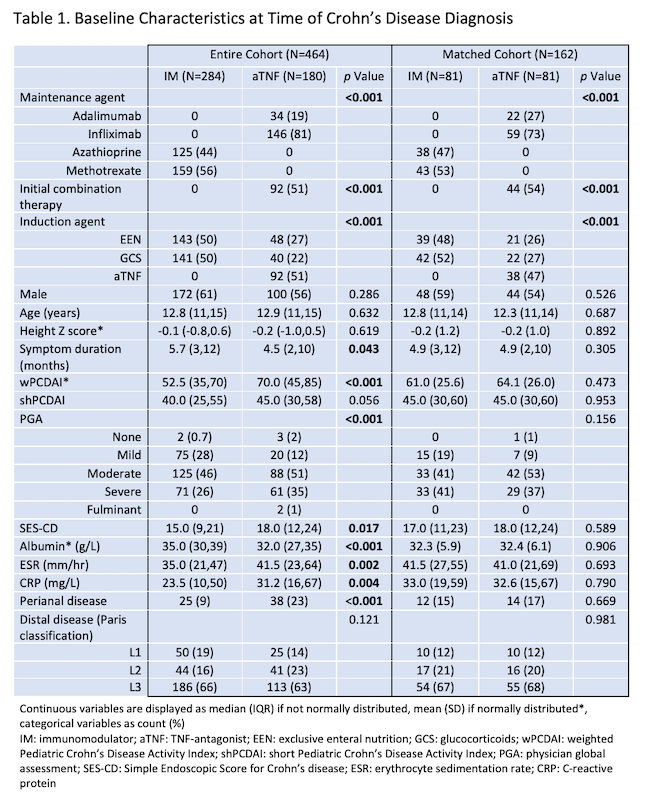

Conclusion
In this paediatric cohort, aTNF was superior to IM as first maintenance therapy. These results may help advocate for better access and funding for early aTNF therapy in paediatric CD.


