P503 The risk of mild, moderate and severe infections in IBD patients: results from a prospective, multicentre, observational cohort study – PRIQ
Rezazadeh Ardabili, A.(1,2)*;van Esser, D.(1);Wintjens, D.(1);Cilissen, M.(1);Deben, D.(3);Mujagic, Z.(1,2);Russ, F.(4);Stassen, L.(2,5);van Bodegraven, A.(4);Wong, D.(3);Winkens, B.(6);Jonkers, D.(1,2);Romberg-Camps, M.(4);Pierik, M.(1,2);
(1)Maastricht University Medical Center+, Department of Internal Medicine- Division of Gastroenterology and Hepatology, Maastricht, The Netherlands;(2)Maastricht University Medical Center+, School for Nutrition and Translational Research in Metabolism NUTRIM, Maastricht, The Netherlands;(3)Zuyderland Medical Centre, Department of Clinical Pharmacy- Clinical pharmacology and Toxicology, Sittard-Geleen, The Netherlands;(4)Zuyderland Medical Centre, Department of Gastroenterology- Geriatrics- Internal and Intensive Care Medicine Co-MIK, Sittard-Geleen, The Netherlands;(5)Maastricht University Medical Center+, Department of Surgery, Maastricht, The Netherlands;(6)Maastricht University, Department of Methodology and Statistics- Care and Public Health Research Institute CAPHRI, Maastricht, The Netherlands;
Background
Immunomodulators and biologicals are essential in current IBD management, but are associated with increased risk of infections. Considering the growing number of treatment options, the benefit-risk balance of drugs is becoming increasingly important in clinical decision making. To date, post-marketing surveillance studies mainly focus on severe infections. As a result, data on mild and moderate infections are scarce. These infections take longer to clear in immunosuppressed patients and can substantially impact quality of life. We aimed to assess the incidence of all infections and identify risk factors for the development of infections in IBD patients.
Methods
We previously developed and validated a Patient-Reported Infections Questionnaire (PRIQ), with excellent diagnostic accuracy, covering 15 infection categories with a 3-month recall period. The current prospective, multicentre, observational cohort study was performed between Jun, 1 2020 and Jul, 1 2021, enrolling consecutive IBD patients using the PRIQ implemented in myIBDcoach, an established telemedicine platform. Infection severity was defined as mild (self-limiting or topical treatment), moderate (oral antibiotics, antivirals or antifungals) or severe (hospitalization or IV treatment). Incidence rates (IR) were calculated for all infections, stratified for severity and subtype. Risk factors for infections were identified using multivariable logistic regression.
Results
In total, 629 IBD patients were included which completed 2391 PRIQs during 572 person-years (PY) of follow-up, resulting in 990 reported infections, corresponding to IRs of 17.3, 11.8, 5.1, and 0.4 per 10PY for all, mild, moderate, and severe infections, respectively (Tables 1-2). Upper respiratory tract (IR 26.9/100PY) and urinary tract infections (IR 14.8/100PY) were the most commonly reported mild and moderate infections (Table 3). Compared to patients without treatment, patients on immunosuppressives more frequently experienced infections of any severity (mild: IR ratio (IRR) 1.57 [95%CI 1.21-2.06] p<0.001, moderate: IRR 1.42 [95%CI 1.20-1.69] p<0.001). On multivariable logistic regression, female sex (mild aOR 1.96; moderate aOR 1.71), smoking status (mild aOR 1.66; moderate aOR 1.86), higher BMI (moderate aOR 1.05), and more comorbidities (mild aOR 2.41; moderate aOR 1.82) were all significantly associated with the development of mild and moderate infections (Table 4).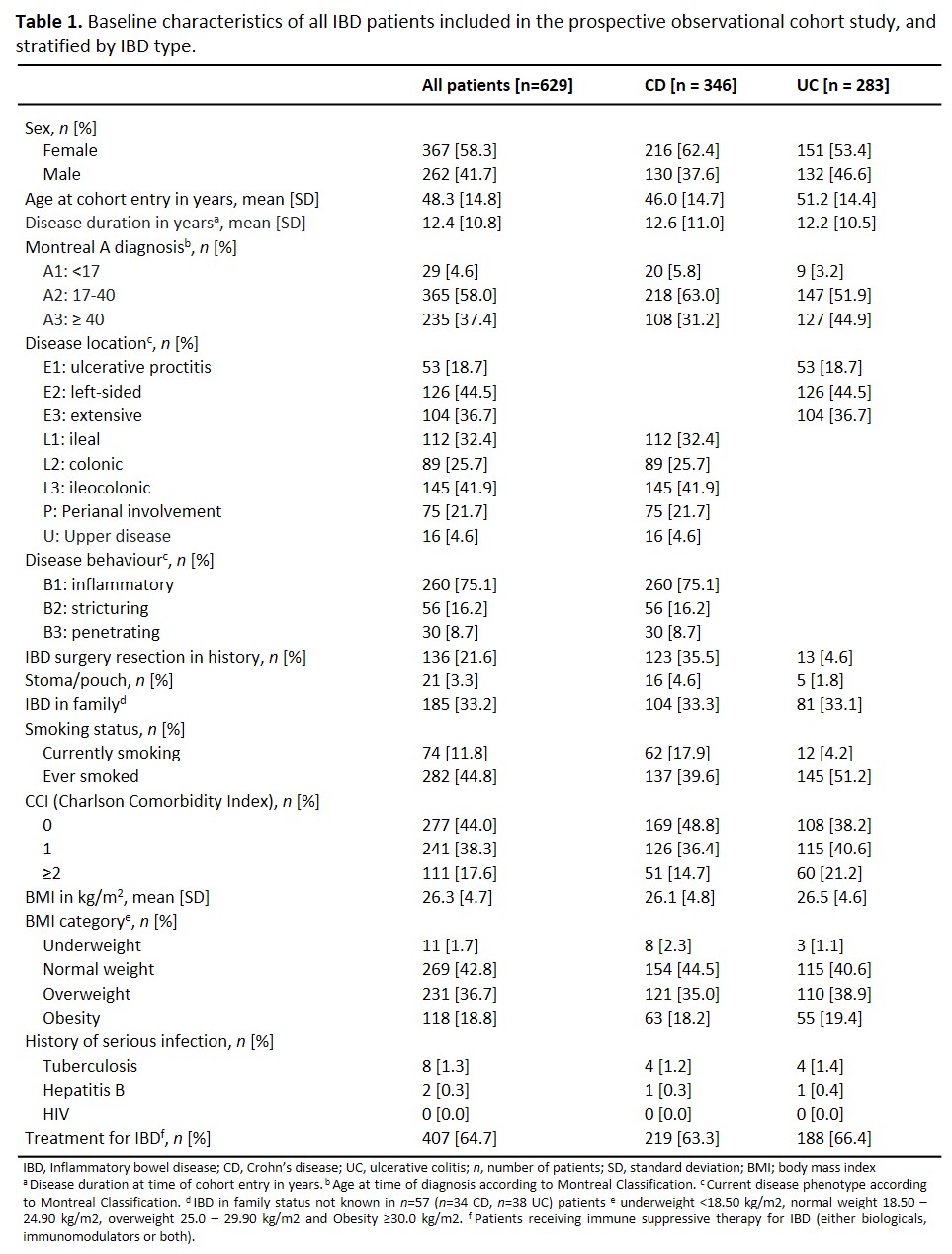



Conclusion
In this real-world prospective study, immune suppressive therapy was associated with mild and moderate infections of any kind in IBD patients. These infections particularly occur in females, smokers, patients with higher BMI and more comorbidities. This information should be considered in personalised treatment selection.


