P515 Comparability of upadacitinib pharmacokinetics in Japanese and non-Japanese subjects with ulcerative colitis with the extended-release formulation
S. Stodtmann1, A. Friedel1, W. Zhou2, M.E. MOHAMED3
1AbbVie, Clinical Pharmacology and Pharmacometrics, Ludwigshafen, Germany, 2Immunology Development, AbbVie, North Chicago, USA, 3Clinical Pharmacology and Pharmacometrics, AbbVie, North Chicago, USA
Background
Upadacitinib (UPA), an oral selective JAK1 inhibitor, is being developed for treatment of patients with moderately-to-severely active ulcerative colitis (UC) in addition to several other inflammatory diseases. UPA has shown favourable efficacy and acceptable safety in UC in an 8-week double-blind placebo-controlled dose-ranging Phase 2b induction study in subjects with moderately-to-severely active UC (U-ACHIEVE trial).
Methods
Sparse blood samples were collected from subjects with UC who were enrolled in the U-ACHIEVE trial, which included Japanese and non-Japanese subjects. UPA plasma concentration vs. time after dose were summarised from the U-ACHIEVE trial. Additionally, data from U-ACHIEVE were pooled with data from UPA Phase 1 and other Phase 2 studies across different inflammatory diseases to characterise UPA population pharmacokinetics. The population pharmacokinetic model was used to estimate UPA plasma exposures from extended-release formulation (dose range 7.5 – 45mg QD) in 168 non-Japanese and 28 Japanese subjects with UC in U-ACHIEVE.
Results
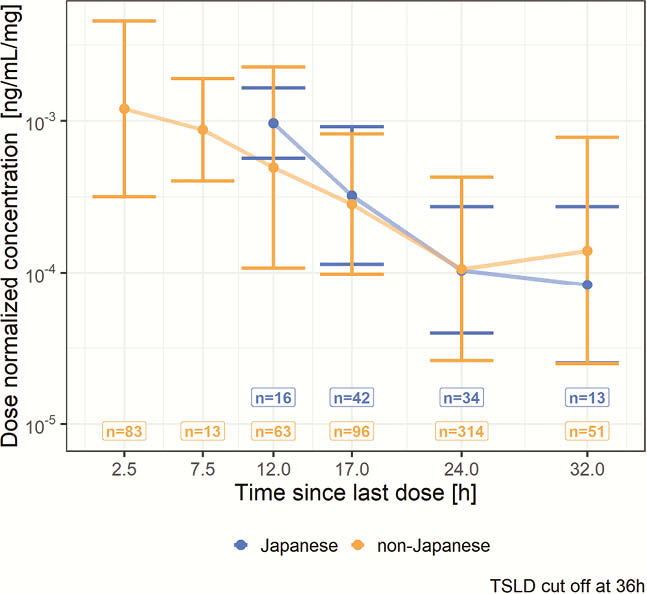
UPA plasma exposures were approximately dose proportional over the evaluated dose range in Japanese subjects with UC. Observed dose-normalised plasma concentrations vs. time since the last dose (Figure 1) and dose-normalised model-estimated UPA exposures were comparable (within 25%) between Japanese and non-Japanese subjects with UC (Table 1).
| Population | Cmax,dn [median (fifth, 95th Percentiles)]ng/ml/mg | Ctrough,dn [median (fifth, 95th Percentiles)]ng/ml/mg | AUCSS,dn [median (fifth, 95th Percentiles)]ng.hr/ml/mg |
| Japanese (N = 28) | 2.98 (2.39 - 4.76) | 0.197(0.05 - 0.47) | 23.0(14.9 - 38.6) |
| Non-Japanese (N = 168) | 2.75(2.00 - 4.58) | 0.159(0.05 - 0.73) | 20.9(14.1 - 44.5) |
Cmax,dn: dose-normalised maximum plasma concentration; AUCSS,dn: dose normalised steady-state AUC; Ctrough,dn: dose-normalised trough plasma concentration.
Conclusion
UPA plasma exposures are similar between Japanese and non-Japanese subjects with UC. This is in agreement with prior assessments in healthy subjects and in subjects with RA which demonstrated comparable UPA exposures between Asian and Western subjects.


