P518 Safety of upadacitinib in ulcerative colitis: Long-term data from the phase 3 open-label extension study (U-ACTIVATE)
Panaccione, R.(1)*;Lichtenstein, G.(2);Nakase, H.(3);Armuzzi, A.(4);Kucharzik, T.(5);Levy, G.(6);Palac, H.(6);Kujawski, M.(6);Klaff, J.(6);Cheon, J.H.(7);
(1)University of Calgary Department of Medicine, Division of Gastroenterology and Hepatology, Calgary, Canada;(2)University of Pennsylvania School of Medicine, Division of Gastroenterology, Philadelphia, United States;(3)Sapporo Medical University School of Medicine, Department of Gastroenterology and Hepatology, Sapporo, Japan;(4)IRCCS Humanitas Research Hospital, IBD Center, Milan, Italy;(5)Klinikum Lüneburg, Division of Gastroenterology and Hepatology, Lüneburg, Germany;(6)AbbVie Inc, Immunology, North Chicago, United States;(7)Yonsei University College of Medicine, Department of Internal Medicine and Institute of Gastroenterology, Seoul, Korea- Republic Of;
Background
Upadacitinib (UPA), an oral reversible and selective JAK inhibitor, has demonstrated efficacy, with a well-characterised safety profile, in two replicate induction studies of UPA 45 mg once daily (QD; U-ACHIEVE induction and U-ACCOMPLISH), and a maintenance study of UPA 15 mg or UPA 30 mg QD (U-ACHIEVE maintenance).1 Here, we report safety data from the ongoing, Phase 3, open-label, 288-week long-term extension (LTE) study in patients with ulcerative colitis (UC) who previously participated in the induction studies.
Methods
Rates of treatment-emergent adverse events (TEAEs) and TEAEs of special interest were calculated by treatment sequence in patients who received ≥1 dose of UPA in the induction studies, maintenance study, or by the data cut-off of 13 December 2021 in the LTE study. Rates are presented as exposure-adjusted event rates (EAERs; events/100 patient-years [PYs]), except for events that typically occur as a single instance, which are presented as exposure-adjusted incidence rates (EAIRs; n/100 PYs). PYs were calculated from the first dose of UPA 45 mg received during induction through to the last UPA dose received or the data cut-off date for ongoing patients.
Results
Overall, 1,308 patients representing 2,350.2 PYs of UPA exposure were evaluated (mean age: 43 years; female: 38%). Across treatment groups, EAERs of serious TEAEs, severe TEAEs, TEAEs leading to UPA discontinuation, TEAEs with reasonable possibility of being related to UPA, or TEAEs leading to death were numerically similar across all UPA treatment sequences (Table 1). EAERs of serious infections, herpes zoster, neutropenia, hepatic disorder, and creatine phosphokinase elevation were numerically higher with UPA 15 mg and 30 mg vs placebo; and creatine phosphokinase elevation with UPA 30 mg vs UPA 15 mg (Table 2). The EAER of anaemia was numerically lower with UPA 30 mg vs UPA 15 mg and placebo. Of note, no remarkable differences were observed in EAIRs of adjudicated major adverse cardiovascular events, adjudicated venous thromboembolic events, or malignancy (excluding non-melanoma skin cancer).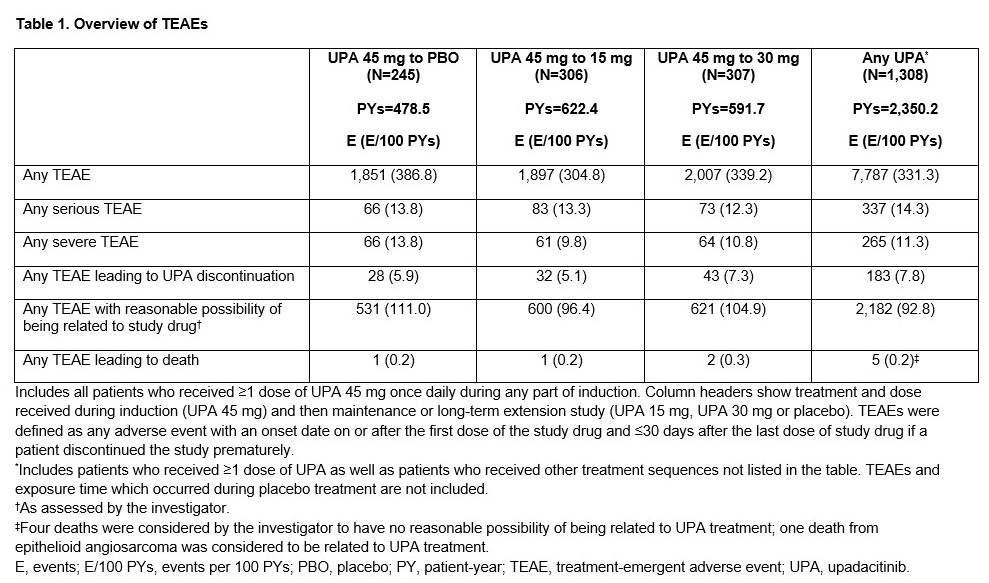

Conclusion
Long-term use of UPA 15 or 30 mg among patients with UC continuing to benefit from prolonged therapy representing 2,350.2 PYs of exposure was well tolerated. The ongoing safety profile of UPA is consistent with previous analyses, with no new safety signals identified compared with previous UC analyses following 52 weeks’ maintenance therapy,1,2 or other immune-mediated indications for which UPA is approved.3,4


