P521 Effectiveness of ustekinumab as therapy for chronic antibiotic refractory pouchitis
Outtier, A.(1)*;Louis, E.(2);Dewit, O.(3);Schops, G.(1);Verstockt, B.(1);Sabino, J.(1);Vermeire, S.(1);Ferrante, M.(1);
(1)UZ Leuven, Gastroenterology, Leuven, Belgium;(2)CHU Liège, Gastroenterology, Liège, Belgium;(3)Cliniques Universitaires Saint-Luc, Gastroenterology, Bruxelles, Belgium;
Background
Up to 10% of patients with ulcerative colitis who undergo a proctocolectomy with ileal pouch-anal anastomosis, will develop chronic antibiotic refractory pouchitis (CARP). As there is a large unmet need in the management of these patients, we evaluated the efficacy and safety of induction and maintenance therapy with ustekinumab (UST).
Methods
We performed a prospective, multicentre, open-label study of patients with CARP (mPDAI ≥5 with an endoscopic subscore ≥2) . Patients received a weight-range-based infusion of UST at baseline (~6mg/kg) and subcutaneous injections of 90mg UST every 8 weeks thereafter until week 48. Patients underwent a pouchoscopy at baseline, week 16 and week 48, with assessment of the modified pouchitis disease activity index (mPDAI). The primary endpoint was the proportion of patients achieving steroid-free remission (mPDAI <5 and reduction by ≥2 points from baseline) at week 16. For this abstract we focus on the secondary endpoints at week 48 including the proportion of patients achieving steroid-free remission and response (reduction of mPDAI by ≥2 points from baseline) using a non-responder imputation. For patients still continuing UST at week 48, we evaluated the change in symptomatic and endoscopic mPDAI subscore, C-reactive protein (CRP) and faecal calprotectin levels compared to baseline. Descriptive statistics and paired nonparametric tests (Wilcoxon signed-rank) of changes from baseline were performed.
Results
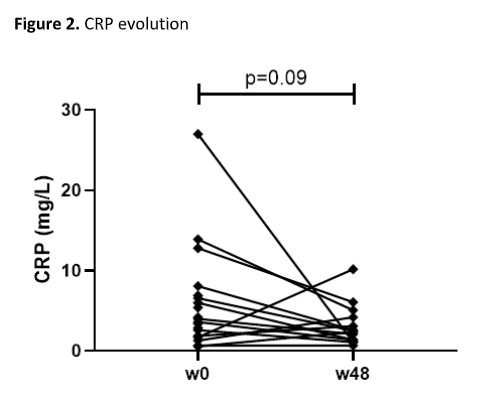
We enrolled 22 patients (59% male, median age 42.2 years, median time after surgery 8.2 years). Twelve (54.5%) patients had previously been treated with biologics for CARP (table 1). At week 16, steroid-free remission was achieved in 27.3% of patients. At week 48, steroid-free remission was achieved in 36.4% and response in 54.5% of patients. In the 14 patients continuing UST at week 48, a significant decrease in total mPDAI (4 (1.8-7.3) vs. 8 (8-10), p<0.001), clinical subscore (1 (0-2.3) vs. 3 (2-4), p=0.001), and endoscopic subscore (3 (1.8-4.3) vs. 6 (4.8-6), p=0.001) was observed compared to baseline (figure 1). Faecal calprotectin (129 (80-344) vs. 229 (139-541) mg/kg, p=0.17) and CRP (2.5 (1.3-4.4) vs. 3.8 (1.6-9.3) mg/L, p=0.09) levels did however not decrease significantly (figure 2 and 3). Three serious adverse events (hospitalization for subobstruction, worsening pouchitis and choledocholithiasis) were recorded, but were not considered related to UST.



Conclusion
In this open-label pilot study in patients with CARP, maintenance therapy with UST showed a clinical and endoscopic effect in half of the patients, but was not associated with changes in inflammatory biomarkers. These promising results warrant confirmation in a placebo-controlled study with UST for the treatment of CARP.


