P522 Prior pharmacotherapy patterns among patients with IBD in the USA initiating biologic therapy
R. Bornheimer1, S. Hass2, A. NAG3, G. Oster1
1Policy Analysis Inc., Health Economics and Outcomes Research, Brookline, USA, 2H. E. Outcomes, Health Economics and Outcomes Research, Los Angeles, USA, 3Shire- a Takeda company, Health Economics- Outcomes Research and Epidemiology, Lexington, USA
Background
Treatment for inflammatory bowel disease (IBD: Crohn’s disease [CD] and ulcerative colitis [UC]) has shifted from symptom management using corticosteroids to targeted therapies such as tumour necrosis factor (TNF) inhibitors. An increase in therapeutic options warrants a better understanding of current treatment pathways. This study reports on the pharmacotherapy history of patients with CD and UC prior to initiation of an IBD-targeted biologic.
Methods
Using the US IQVIA™ Real-World Data Adjudicated Claims Database, we identified adults (≥18 years) with CD or UC who initiated biologics indicated for these diseases from 1 January 2016 to 30 June 2017. Patients were stratified based on the most recent biologic received; the date of initiation was designated the index date. Patients with < 12 months of continuous medical coverage prior to the index date were excluded. Patterns of prior IBD-related pharmacotherapy, in terms of the number and type of biologics, and time spent receiving the biologic, were examined in relation to the index biologic for the study period 1 January 2011 to 30 June 2017.
Results
In total, 3010 patients with UC and 5210 with CD were identified. Among patients with UC, 1285, 977, 587 and 161 initiated treatment with adalimumab, infliximab, vedolizumab and golimumab, respectively. Among patients with CD, 2634, 1436, 737, 241 and 162 began treatment with adalimumab, infliximab, vedolizumab, certolizumab and ustekinumab, respectively. Most patients beginning treatment with infliximab or adalimumab were biologic-naïve (UC: 79.9%, 89.3%, respectively; CD: 77.8%, 84.6%, respectively). Patients starting treatment with other biologics, however, had typically received at least one IBD-related biologic previously (Figure 1). Among patients who were not biologic-naïve at initiation of therapy, the mean total duration of prior biologic exposure was 17.8 months and 26.6 months for patients with UC and CD, respectively.
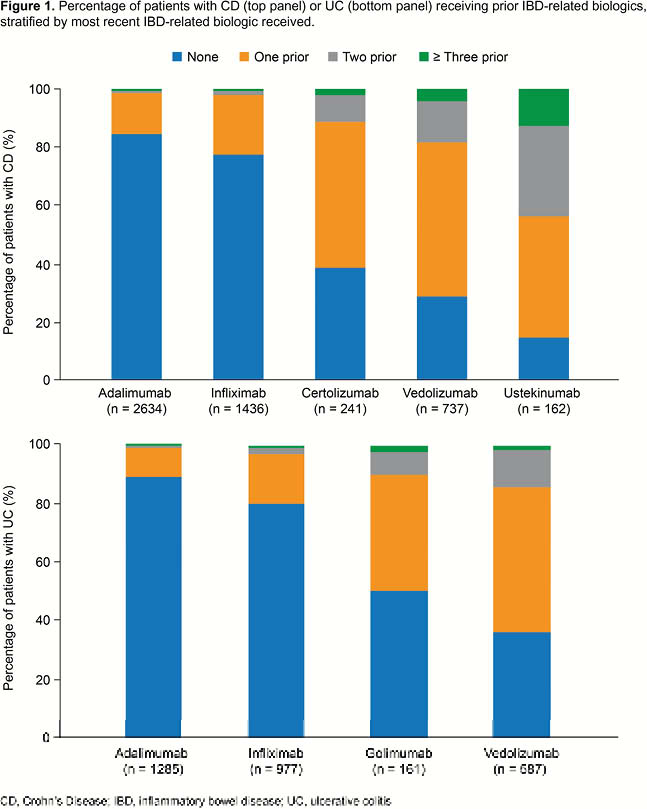
Conclusion
During the study period, anti-TNFs were the most commonly initiated biologics in both groups of patients. Although newer IBD-related biologics (i.e. vedolizumab and ustekinumab) are typically preceded by treatment with other biologics, most patients receiving an anti-TNF are biologic-naïve.


