P524 Compared efficacy of ustekinumab and anti-TNF agents as first-line biological therapy in luminal Crohn's disease.
Riviere, P.(1);Kanters, C.(2);Ni, A.(2);Pellet, G.(1);Hupe, M.(1);Aboulhamid, N.(2);Poullenot, F.(1);Bitton, A.(2);Zerbib, F.(1);Lakatos, P.(2);Afif, W.(2);Laharie, D.(1);Bessissow, T.(2);
(1)Bordeaux University Hospital, Gastroenterology unit, Pessac, France;(2)McGill University Health Centre, Gastroenterology, Montréal, Canada;
Background
Real-life data on the efficacy of ustekinumab as first-line therapy for the treatment of moderate to severe Crohn’s disease (CD) is lacking. The objective of this study was to compare the clinical remission rate at 3 months of patients treated by ustekinumab or anti-TNF agents as first-line biological therapy.
Methods
We conducted a two-center retrospective study including biologic-naïve patients starting either ustekinumab or an anti-TNF agent for CD between January 2016 and December 2019. The primary endpoint was clinical remission at 3 months after therapy initiation, defined as a Harvey Bradshaw Index (HBI) < 4 without steroids, need for CD-related surgery or treatment discontinuation due to treatment failure or intolerance. Secondary endpoints were remission at 12 months and time to drug withdrawal. Patients treated with ustekinumab were matched 1:3 to patients receiving anti-TNF agents using a propensity score including age, CD duration, smoking status, CD location according to the Montréal classification and history of perianal disease.
Results
208 patients [104 (50%) female, median age (InterQuartileRange) 30 years (23-47)] were included, 156 (75%) patients starting anti-TNF (95 (45%) adalimumab and 61 (29%) infliximab) and 52 (25%) ustekinumab and followed for a median (IQR) duration of 39 (22-52) months. Median (IQR) CD duration was 21 (6-104) months and 45 (22%) patients had previously undergone a CD-related surgery. At inclusion, the median (IQR) HBI was 5 (2-8) and 83 (39%) patients were receiving oral corticosteroids.
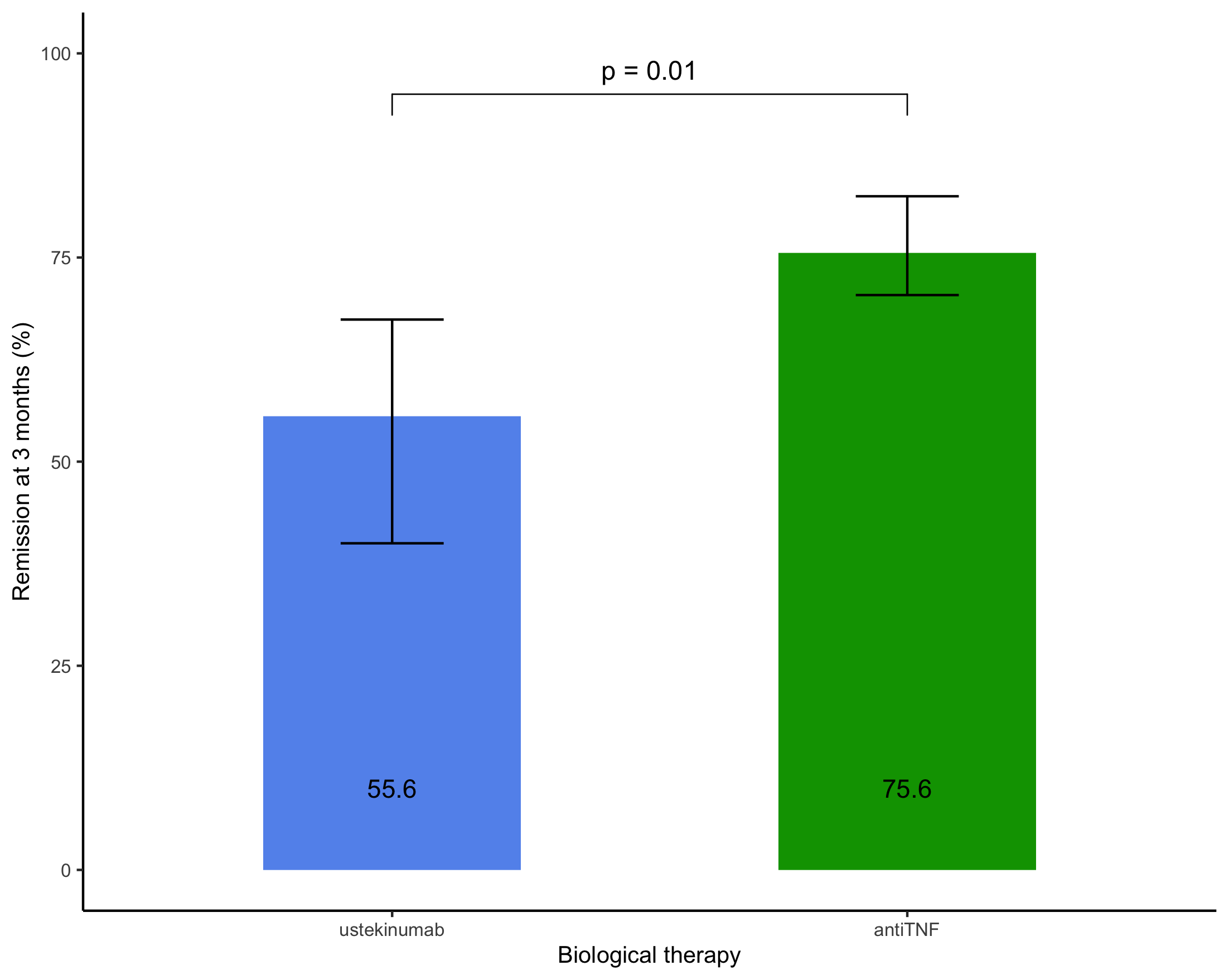
Conclusion
In this retrospective cohort, we found that anti-TNF agents as a first-line biological therapy led to higher rates of remission at 3 and 12 months in biologic-naïve patients with Crohn's disease than ustekinumab.


