P524 Predictors of immunogenicity in patients with Inflammatory Bowel Disease treated with infliximab: A post hoc analysis of the randomised phase I CT-P13 SC study
Schreiber, S.(1);Ben-Horin, S.(2);Ye, B.D.(3);Kim, D.H.(4);Reinisch, W.(5)*;
(1)University Hospital Schleswig-Holstein, Department for Internal Medicine I, Kiel, Germany;(2)Tel Aviv University, Gastroenterology Department- Chaim Sheba Medical Center, Tel-Hashomer, Israel;(3)University of Ulsan College of Medicine, Department of Gastroenterology and Inflammatory Bowel Disease Center- Asan Medical Center, Seoul, Korea- Republic Of;(4)Celltrion Healthcare Co., Ltd, Incheon, Korea- Republic Of;(5)Medical University of Vienna, Department of Internal Medicine III- Division of Gastroenterology and Hepatology, Vienna, Austria;
Background
CT-P13 SC is the only subcutaneous (SC) formulation of infliximab (IFX) approved to treat Crohn’s disease (CD) or ulcerative colitis (UC). The primary CT-P13 SC study reported no increased risk of immunogenicity (anti-drug antibody [ADA]/neutralising antibody [NAb] positivity) with CT-P13 SC vs. intravenous (IV) in CD or UC patients (pts) despite a numerical trend for lower ADA/NAb positivity with SC dosing.1 It is unclear whether high trough levels (Ctrough) during IFX dosing may account for these observations.
Methods
This post hoc analysis of part 2 of the randomised, parallel-group CT-P13 SC study (NCT02883452) evaluated predictors of immunogenicity in CD or UC pts treated with CT-P13. The relationship of Week (W) 22 and 30 ADA and NAb positivity rates with W22 CT-P13 Ctrough, under stable dosing, was examined. The optimal threshold of Ctrough for predicting ADA positivity was estimated by receiver operating characteristic (ROC) methodology using W22 data. A generalised linear regression model identified predictive factors for ADA and NAb positivity at W22 and W30. The proportion of pts with Ctrough levels equal to or above the calculated predictive threshold, and ADA/NAb positivity rates, were determined by treatment arm.
Results
The analysis included 131 pts randomised at W6 to receive CT-P13 SC (n=66) or CT-P13 IV (n=65) as maintenance treatment. Of 113 pts with both Ctrough and immunogenicity data available at W22, 60 were ADA negative, and 53 were ADA positive. At W22, median Ctrough levels were significantly higher in the ADA-negative vs. ADA-positive group (16.95 vs. 3.44 μg/mL, p<0.0001); ADA positivity differed significantly according to Ctrough quartile (p=0.0009; Fig. 1).
ROC analysis showed that a Ctrough threshold of 4.695 μg/mL optimally predicted ADA positivity with 66.2% sensitivity and 77.3% specificity (area under the ROC curve, 0.766 [95% confidence interval, 0.664–0.869]; Fig. 2).
Concomitant medication use and W22 Ctrough ≥4.695 μg/mL were significantly associated with lower rates of ADA and NAb positivity at W22 and were significant predictors of lower rates of ADA and NAb positivity at W30 (Fig. 3).
At W22, a higher proportion of pts had Ctrough ≥4.695 μg/mL in the SC vs. IV arm (96.5% vs. 25.0%, p<0.001). In the SC vs. IV arm, the proportion of pts with ADA and NAb positivity was significantly lower at W22 (ADA, 36.8% vs. 57.1%, p=0.039; NAb, 7.0% vs. 21.4%, p=0.033; Fig. 4).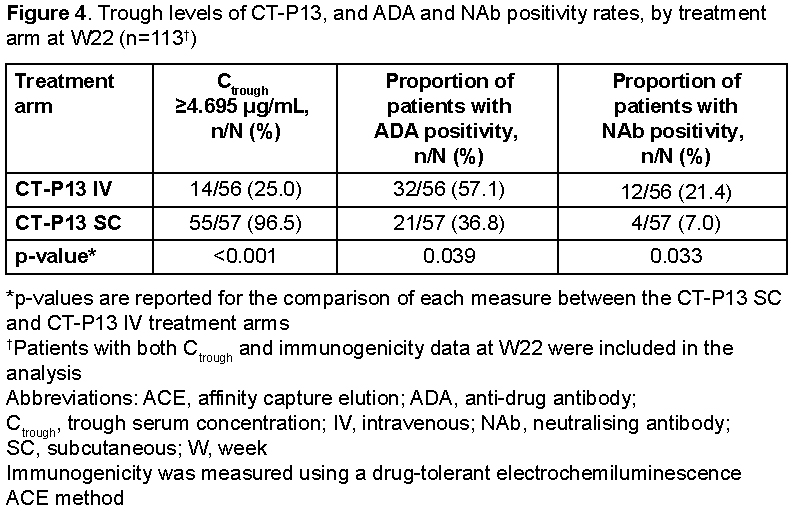
Conclusion
Ctrough level predicted ADA and NAb positivity in CD or UC pts, with levels ≥4.695 μg/mL significantly associated with lower rates of positivity, providing initial evidence on high zone tolerance of IFX.
Reference
1. Schreiber S et al. Gastroenterology 2021;160:2340–2353.


