P526 A prediction model for successful increase of adalimumab dose intervals: analysis of the pragmatic open-label randomised controlled non-inferiority LADI trial
Van Linschoten, R.(1,2)*;Jansen, F.(3);Pauwels, R.(2);Smits, L.(3);Atsma, F.(4);Kievit, W.(5);de Jong, D.(3);de Vries, A.(2);Boekema, P.(6);West, R.(1);Bodelier, A.(7);Gisbertz, I.(8);Wolfhagen, F.(9);Romkens, T.(10);Lutgens, M.(11);van Bodegraven, A.(12);Oldenburg, B.(13);Pierik, M.(14);Russel, M.(15);de Boer, N.(16);Mallant-Hent, R.(17);ter Borg, P.(18);van der Meulen-de Jong, A.(19);Jansen, J.(20);Jansen, S.(21);Tan, A.(22);van der Woude, C.J.(2);Hoentjen, F.(23);
(1)Franciscus Gasthuis & Vlietland, Gastroenterology & Hepatology, Rotterdam, The Netherlands;(2)Erasmus MC, Department of Gastroenterology & Hepatology, Rotterdam, The Netherlands;(3)Radboud University Medical Center, Department of Gastroenterology and Hepatology, Nijmegen, The Netherlands;(4)Radboud University Medical Center, IQ Healthcare, Nijmegen, The Netherlands;(5)Radboud University Medical Center, Radboud institute for Health Science- Department for Health Evidence, Nijmegen, The Netherlands;(6)Maxima Medical Center, Department of Gastroenterology and Hepatology, Eindhoven, The Netherlands;(7)Amphia Hospital, Department of Gastroenterology and Hepatology, Breda, The Netherlands;(8)Bernhoven Hospital, Department of Gastroenterology and Hepatology, Uden, The Netherlands;(9)Albert Schweitzer Hospital, Department of Gastroenterology and Hepatology, Dordrecht, The Netherlands;(10)Jeroen Bosch Hospital, Department of Gastroenterology and Hepatology, 's-Hertogenbosch, The Netherlands;(11)Elisabeth Tweesteden Hospital, Department of Gastroenterology and Hepatology, Tilburg, The Netherlands;(12)Zuyderland Medical Center, Dept. of Gastroenterology- Geriatrics- Internal and Intensive Care Medicine Co-MIK, Sittard-Geleen/Heerlen, The Netherlands;(13)University Medical Center Utrecht, Department of Gastroenterology and Hepatology, Utrecht, The Netherlands;(14)Maastricht University Medical Center+, Department of Gastroenterology and Hepatology, Maasstricht, The Netherlands;(15)Medisch Spectrum Twente, Department of Gastroenterology and Hepatology, Twente, The Netherlands;(16)Amsterdam University Medical Center- Vrije University Amsterdam, Department of Gastroenterology and Hepatology- AGEM research institute, Amsterdam, The Netherlands;(17)Flevoziekenhuis, Department of Gastroenterology and Hepatology, Almere, The Netherlands;(18)Ikazia Hospital, Department of Gastroenterology and Hepatology, Rotterdam, The Netherlands;(19)Leiden University Medical Center, Department of Gastroenterology and Hepatology, Leiden, The Netherlands;(20)OLVG, Department of Gastroenterology and Hepatology, Roterdam, The Netherlands;(21)Reinier de Graaf Gasthuis, Department of Gastroenterology and Hepatology, Delft, The Netherlands;(22)Canisius Wilhelmina Hospital, Department of Gastroenterology and Hepatology, Nijmegen, The Netherlands;(23)University of Alberta, Division of Gastroenterology- Department of Medicine, Edmonton, Canada; on behalf of the LADI study group and the Dutch Initiative on Crohn and Colitis (ICC)
Background
We showed in the pragmatic open-label randomised controlled non-inferiority LADI trial that increasing adalimumab (ADA) dose intervals was non-inferior to conventional dosing for persistent flares in CD patients in stable remission, while infection-related adverse events (AE) were reduced. This was counterbalanced by lower rates of clinical remission and more gastro-intestinal AEs after 48 weeks. In the current study we aimed to develop a prediction model to identify patients who could successfully increase their ADA dose interval.
Methods
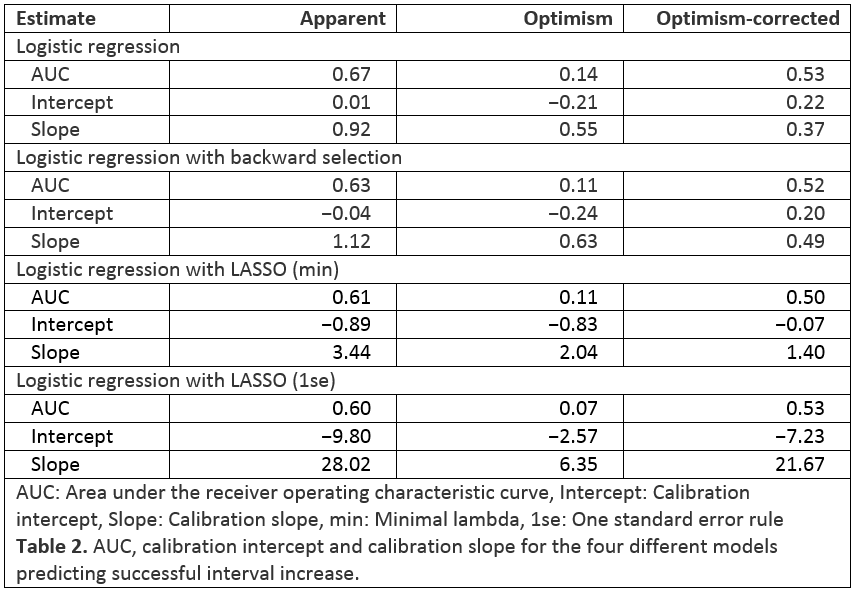
This is a secondary analysis of the intervention group of the LADI trial. In this group, patients in steroid-free clinical remission for ≥ 9 months (Harvey-Bradshaw Index (HBI) < 5, CRP < 10 mg/L and faecal calprotectin (FCP) < 150 µg/g), on conventional ADA dosing increased ADA intervals to 3 and then to 4 weeks. A successful dose interval increase was defined as: no persistent flare (>8 weeks), no intervention-related severe AE, no rescue medication use, and an increased dose interval while in clinical and biochemical remission at week 48. Candidate baseline predictors were selected after a study group consensus meeting (Table 1). Prediction models were based on logistic regression. Four variable selection strategies were used: inclusion of all variables as a naïve reference model, stepwise backwards regression, LASSO with minimal lambda, and LASSO using the ‘one standard error’ rule. Models were evaluated on discrimination and calibration. Missing data were multiply imputed and models were internally validated using bootstrap optimism correction.
Results
The four models were developed on 109 patients, of which 59.1% experienced the outcome of successful dose interval increase (Table 1). Apparent performance of the models was adequate, with areas under the receiver operating characteristic curves (AUC) between 0.60 and 0.67 (Table 2 and Figure 1). Predicted probabilities from LASSO-based models were too modest (Table 2 and Figure 2). Internal validation showed optimism-corrected AUCs around 0.5 (Table 2), meaning that models could not identify patients who successfully increased their ADA dose interval. Optimism-corrected calibration estimates for the naïve model and backwards selection showed overfitting on the data. Internally validated predicted probabilities from LASSO-based models were too modest.

Conclusion
After 48 weeks, approximately 60% of patients could successfully increase their ADA dose interval without major negative clinical impact, but these could not be identified with a series of different prediction models. Risks and benefits of this strategy should be discussed with individual patients based on their risk perception and medication preferences.