P533 Adalimumab 80mg every other week in inflammatory bowel disease: Treatment intensification outcomes in real life clinical practice
Calvo Moya, M.I.(1);Omella Usieto, I.(2);El Hajra Martinez, I.(2);Santos Perez, E.(2);Gonzalez Lama, Y.(1);Ruiz Antoran, B.(3);Menchen Viso, B.(4);Matallana Royo, V.(1);Gonzalez Partida, I.(1);De Lucas Tellez de Meneses, R.(1);Bella del Castillo, P.(1);Gonzalez Rodriguez, M.(1);Vera Mendoza, M.I.(1);
(1)Hospital Universitario Puerta de Hierro - Majadahonda, IBD Unit. Gastroenterology and Hepatology, Madrid, Spain;(2)Hospital Universitario Puerta de Hierro - Majadahonda, Gastroenterology and Hepatology, Madrid, Spain;(3)Hospital Universitario Puerta de Hierro - Majadahonda, Clinical Pharmacology, Madrid, Spain;(4)Hospital Universitario Puerta de Hierro - Majadahonda, Hospital Pharmacy, Madrid, Spain
Background
Adalimumab (ADA) intensification is recommended for inadequate or loss of response in inflammatory bowel disease (IBD) patients. A new presentation of ADA 80mg administered every other week (eow) has been approved as an alternative to ADA 40mg every week (ew). Data regarding impact of ADA 80mg eow in clinical practice is still scarce. The aim of this study was to assess long-term durability, safety and cost-effectiveness of treatment with ADA 80mg eow in patients with IBD.
Methods
A retrospective cohort study in a tertiary hospital that included all IBD patients under intensified maintenance therapy with ADA 80mg eow was performed. Durability was calculated considering the time from the first dose to treatment withdrawn or to the end of follow-up. Biological remission (BR) was defined as CR together with fecal calprotectin (FC) <250µg/g and C-reactive protein (CRP) <5mg/dl. Economic impact of ADA 80mg eow was estimated considering current price of both ADA 40mg and ADA 80mg pens at our centre.
Results
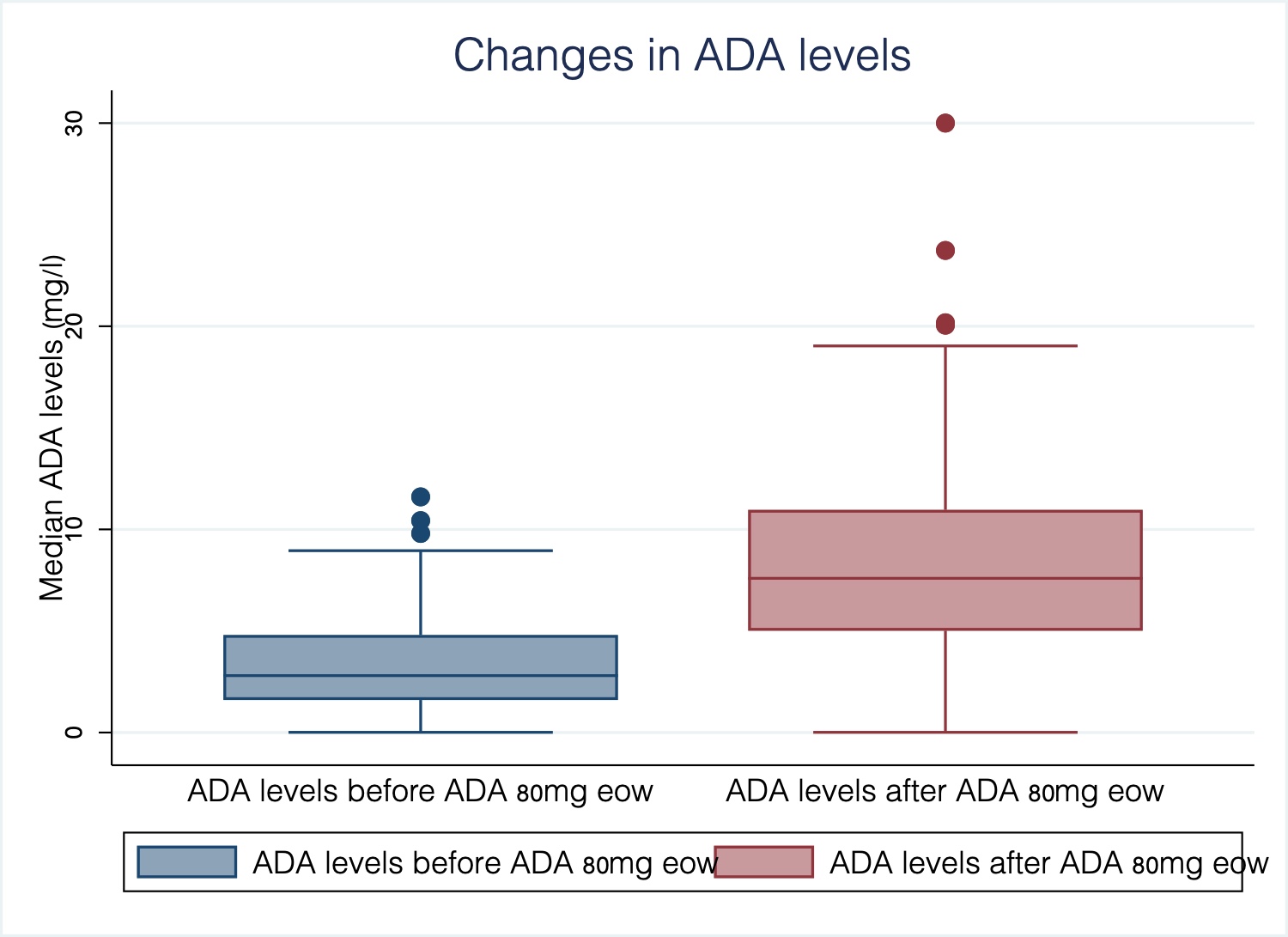
Sixty-three patients (52 CD and 11 CU) were included; median age 47 (IQR 39-59), 54% male; median duration of the disease before ADA of 11 years (IQR 6-20); 30% were active smokers. Among CD patients, 56% had ileal disease, 17% colonic and 27% ileocolonic. The inflammatory behavior was the most frequent (52%) and 31% had perianal disease. In UC, 55% had extensive colitis. 44 patients (70%) were bio-naïve and 36 (57%) received immunosuppressants at baseline. At the time of escalation, 48 patients (76%) were symptomatic. After intensification, 52 (83%) patients (CD 42 and UC 10) achieved CR and 46 (73%) BR. The changes in the levels of FC, CRP and ADA were significant (p <0.001) (Graphs 1-3).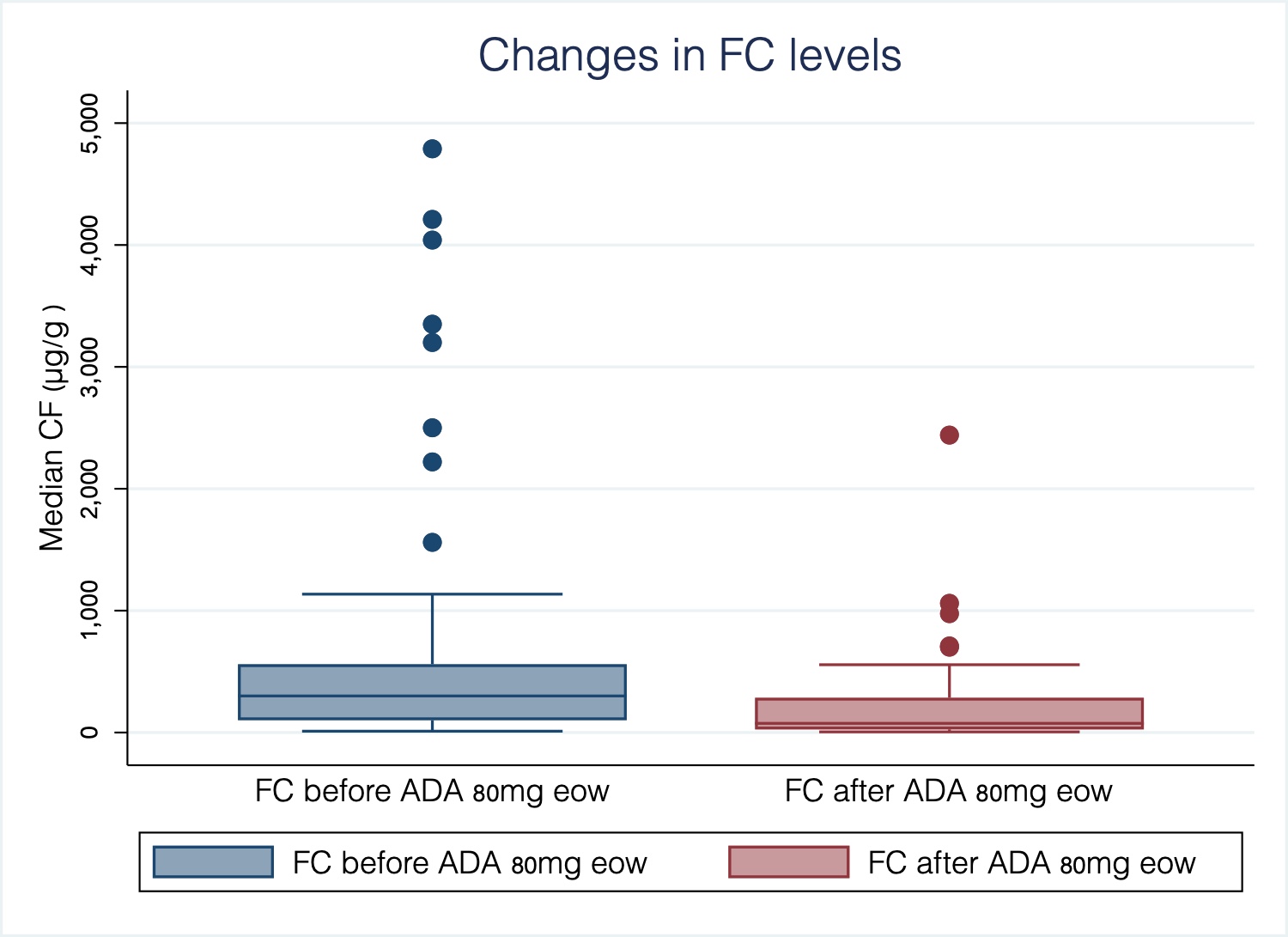

22 patients (35%) discontinued treatment after a median of 6.5 (IQR 5-10) months due to: 11 no clinical response (50%), 4 loss of response (18%), 3 adverse events (14%) (psoriasis) and 4 endoscopic progression (18%). 44 patients (70%) remained under treatment and in CR (median follow-up 17 months, IQR 13-24) (Graph 4) and with a median ADA levels of 10.46 mg/l (IQR 7.34-15.25). Use of ADA 80 eow regimen saved 223500€ in patients who maintained treatment. In the multivariate analysis, being in CR when intensifying reduced the risk of treatment discontinuation by 87% (HR 0.13, 95%CI 0.02-0.99; p<0.001), having reached BR by 99.5% (HR 0.05, 95%CI 0.02-0.14; p <0.001) and having ADA levels ≥5 mg/l after intensification by 68% (HR 0.32, 95%CI 0.13-0.75; p = 0.02). Smoking habit was associated with treatment withdrawn (HR 1.74, 95%CI 1.02-2.96; p=0.04).
Conclusion
ADA intensification to 80mg eow in IBD patients is safe, effective and may reduce costs in real life clinical practice. Early intensification, even in CR, may enhance ADA treatment durability.


