P535 Optimising vedolizumab in treatment sequences for Crohn’s disease: Results from a simulation model using real-world evidence
Louis, E.(1);Nikolaou, A.(2);Litkiewicz, M.(2);Agboton, C.(3);Wang, S.(4);Armuzzi, A.(5);
(1)University Hospital CHU of Liège, Department of Gastroenterology, Liège, Belgium;(2)Evidera, Modelling and Simulation, London, United Kingdom;(3)Takeda, Global Medical Affairs - Gastroenterology, Zurich, Switzerland;(4)Takeda, Global Evidence & Outcomes - Gastroenterology, Cambridge, United States;(5)Fondazione Policlinico Universitario A. Gemelli IRCCS - Università Cattolica, Gastroenterology Unit CEMAD, Rome, Italy
Background
With the availability of biologic therapies for treating Crohn’s disease (CD) there are limited data to inform optimal treatment decisions. Vedolizumab (VDZ), an anti-integrin antibody that selectively targets lymphocyte migration to the gut, was approved in 2014 for the treatment of moderate-to-severe CD. We developed a model based on real-world evidence (RWE) to examine the effect of different positioning for VDZ in CD treatment sequences on clinical outcomes.
Methods
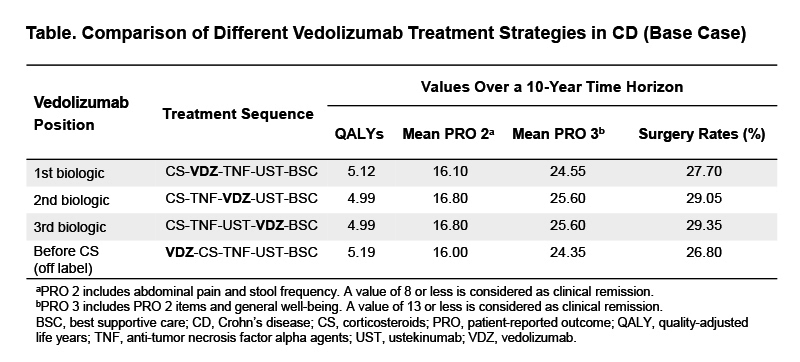
A Markov sequential model was developed to identify the optimal position of VDZ within a sequence of CD therapies that included corticosteroids (CS), 2 biologics and best supportive care. Three sequences were compared: VDZ as first biologic (first position), second and last biologic among anti-TNFs and ustekinumab as alternate biologic treatments. To test the behaviour of the model, VDZ was also positioned before CS (Position 0, off label). Model inputs were informed using published RWE. At each position in the treatment sequence, patients achieving clinical response or remission during induction continued on maintenance; patients on maintenance could lose clinical response or remission; patients who did not achieve response during induction or lost response during maintenance (including after dose escalation) moved to the next position of the sequence after a treatment-free interval. Surgery, development of intestinal malignancy/lymphoma, and discontinuation of treatment due to serious adverse events or death could occur at each sequence position. VDZ sequences were compared and ranked based on quality-adjusted life years (QALYs), patient-reported outcomes (PRO2/3) derived from CDAI scores, or proportion of patients undergoing surgery by the end of the 10-year time horizon for model simulation. Probabilistic sensitivity analysis (PSA) evaluated the impact of model inputs uncertainty on the outcomes.
Results
For QALYs, PRO2/3, and rates of surgery, VDZ early in the treatment sequence was the optimal positioning strategy (Table). Using QALYs as the ranking criterion, VDZ before CS was the optimal sequence yielding 5.19 QALYs and incremental QALYs of 0.7, 0.20, and 0.20 versus the second-, third- and fourth-line strategy, respectively. The ranking of VDZ in treatment sequences was preserved when PRO2/3 or surgery were used as ranking criteria. In 1,780/2,000 PSA iterations (89%), VDZ before CS or as first biologic yielded the highest QALYs vs. other sequence positions.
Conclusion
The results of this simulation model using real-world evidence clearly indicate that positioning VDZ earlier in the treatment sequence may yield better patient outcomes in terms of QALYs, PROs and rates of surgery.