P544 Point-of-care finger prick test for C-reactive protein, infliximab and adalimumab serum concentrations
Volkers, A.(1);Löwenberg, M.(1);Bray, K.(2);Bahur, B.(2);Braad, M.(1);Abeling, Y.(1);Gecse, K.(1);Duijvestein, M.(1);Ponsioen, C.(1);D'Haens, G.(1);
(1)AmsterdamUMC, Gastroenterology and Hepatology, Amsterdam, The Netherlands;(2)ProciseDx, Clinical Dev & Medical Affairs, San Diego, United States
Background
Point-of-Care tests (POCTs) allow instant measurement of inflammatory markers and/or drug concentrations. However, currently available POCTs for infliximab (IFX) and adalimumab (ADL) serum concentrations are time consuming. Recently, a new POCT device (ProciseDx, San Diego, CA, USA) was developed which conveniently measures C-reactive protein (CRP), IFX and ADL capillary concentrations within minutes. We aimed to validate this device by comparing POCT results with conventional laboratory tests for serum CRP, IFX and ADL in patients with inflammatory bowel disease (IBD).
Methods
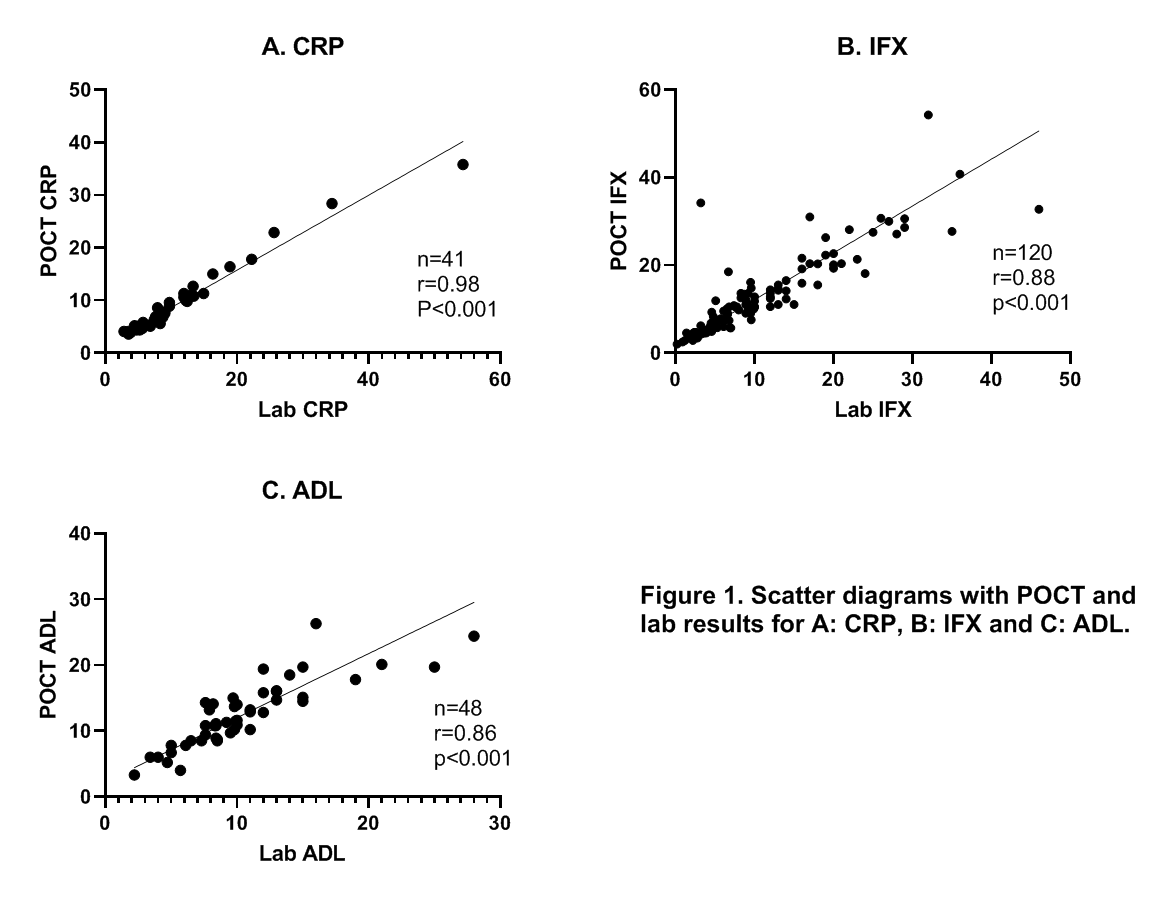
IBD patients requiring routine measurement of serum CRP, IFX or ADL were invited to participate. Along with serum collection, 20μl of capillary blood was obtained via finger prick and dispensed in a cartridge with a buffer, and placed in the POCT device providing results within two to four minutes. Forty patients were needed to validate the CRP POCT, as this assay was already previously validated in a different population. For the IFX and ADL assays, 120 patients using IFX or ADL were required to validate the POCT. Agreement between the laboratory serum assay and POCT was visualized on a scatter diagram and a Bland-Altman plot. Deming regression was calculated to demonstrate agreement. In addition, Pearson’s correlation coefficient was calculated.
Results
Until now, 41 patients have been enrolled for the CRP assay, 120 patients for the IFX and 46 patients for the ADL assay. Two ADL patients were sampled twice (n=48). Significant correlations were found for CRP, IFX and ADL (r=0.98, r=0.88 and r=0.86 respectively, Fig. 1). Deming regression analysis of the CRP assay resulted in a slope of 0.71 (95% CI 0.49 to 0.93) and 1.5 (95% CI -0.44 to 3.50) for the intercept. For the IFX assay, the slope was 1.1 (95% CI 0.83 to 1.3) and the intercept was 1.4 (95% CI -0.47 to 3.4). For the ADL assay, the slope was 0.97 (95% CI 0.64 to 1.3) and the intercept was 2.3 (95% CI -0.64 to 5.2).
Conclusion
A novel POCT using a finger prick approach provides a rapid, user friendly and reliable measurement of CRP, IFX and ADL concentrations within minutes. The capillary CRP was slightly lower than the venous serum CRP, which was consistently observed and considered clinically irrelevant in this cohort.