P545 Calprotectin at week 12 is a predictor for histological remission in ulcerative colitis patients treated with Vedolizumab
Rubstov, D.(1);Kakkadasam Ramaswamy, P.(1);Edwards, J.(1);Shukla, D.(1);Willmann, L.(1);Moattar, H.(1);Bhullar, M.(1);Ishaq, N.(1);Dorrington, A.(1);Mohsen, W.(1);
(1)Gold Coast University Hospital, Department of Digestive Health, Southport, Australia
Background
Vedolizumab (VDZ) is a gut-specific α4β7 integrin antagonist that has demonstrated efficacy for induction and maintenance of remission in moderate to severe ulcerative colitis (UC). The aim of this study was to assess the rates of histological remission (HR) in a real-world setting and to identify predictors for histological remission.
Methods
Retrospective cohort study of all UC patients (≥18 years) initiated on VDZ from 2016 to 2020 was completed. Clinical, biochemical, endoscopic and histologic data were collected. All patients received standard induction therapy with VDZ 300 mg IV at Weeks 0, 2, and 6 and maintained on an 8-weekly regimen. Dose was escalated to a every 4-weekly regimen as per physician’s discretion. A 52-week follow-up was completed on all patients. Endoscopic assessment was carried out between 24 and 52 weeks after commencing VDZ. Histological activity was graded as per Nancy index and histological remission was defined as Nancy grade 0. Endoscopic remission was defined as Mayo endoscopic score = 0. Clinical remission was defined as SCCAI ≤ 5.
Results
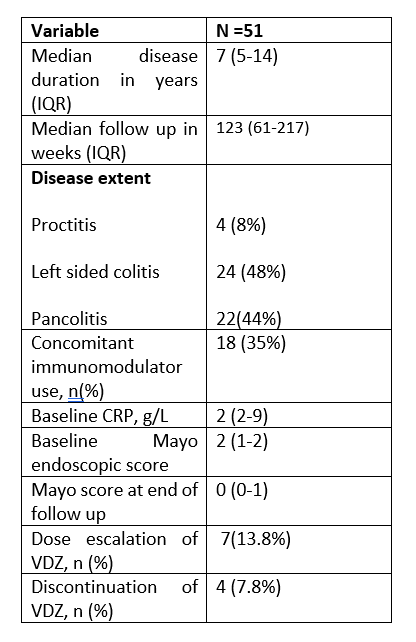
A total of 51 patients [55% female, median age 48 years (IQR 35-60)] were included. 16/51 (34%) were anti-TNF exposed. In 30/51 (59%) patients VDZ was combined with steroids at induction and by week 12 steroids were completely tapered in 14/30 (46.7%) patients. At weeks 12, 24 and 52, 89.6%, 87% and 97.5% of patients, respectively, were in clinical remission. 19/37 (51.3%) patients were in endoscopic remission at end of follow up. Median Nancy score prior to commencing VDZ was 3 (IQR: 2-4) and the median Nancy score at end of follow up was 1 (IQR: 0-2). 19/37 (51.3%) patients achieved HR; 3 patients who were in HR at the time of commencement of VDZ remained in HR at the end of follow up. Median baseline faeces calprotectin (FC) was 320 mcg/g (IQR 45-1000) and was similar in patients who achieved HR and those who did not. Median FC at 12 weeks was 155 mcg/g (45-720) and was significantly lower in patients who achieved HR when compared to patients who did not achieve histological remission (45 vs 420, p 0.028). FC at week 12 predicted histological remission (AUC =0.8667). FC ≥ 200mcg/g at week 12 predicted failure to achieve HR with sensitivity 70%, specificity 100%, PPV 100%, NPV 75%, accuracy 84%.
Conclusion
Vedolizumab is effective in achieving histological remission and FC ≥ 200 mcg/g at week 12 accurately predicts failure to achieve HR in patients treated with VDZ.