P545 Safety of ustekinumab in pregnant patients with inflammatory bowel disease and in their offspring: results from the DUMBO registry of GETECCU
Chaparro, M.(1);Gutiérrez, A.(2);Calviño-Suárez, C.(3); Huguet, J.M.(4);Calvo, M.(5);Aguas, M.(6);Camargo Camero, R.(7);de Jorge Turrión, M.Á.(8);Hervías Cruz, D.(9);López Serrano, P.(10);Marín Pedrosa, S.(11);Martínez Montiel, P.(12);Rivero, M.(13);Vicente Lidón, R.(14);Arias García, L.(15);Arroyo, M.(16);Bujanda, L.(17); Casanova, M.J.(1);Figueiras, M.(18);Lucendo, A.J.(19);Manceñido Marcos, N.(20);Márquez, L.(21);Martín-Arranz, M.D.(22);Boscá Watts, M.(23);Ber, Y.(24);Ramírez de la Piscina Urraca, P.(25);Pérez-Martínez, I.(26);Robles, V.(27);Ruiz-Cerulla, A.(28);Vázquez Morón, J.M.(29);Madero, L.(2);Barreiro-de Acosta, M.(3);Capilla, M.(4);Vera Mendoza, I.(5);Acosta, D.(1);Brenes, Y.(1);Hermida, S.(1);Parra, P.(1);G. Donday, M.(1); Gisbert, J.P.(1);
(1)Hospital Universitario de La Princesa- Instituto de Investigación Sanitaria Princesa IIS-IP and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas CIBEREHD, Gastroenterology Unit, Madrid, Spain;(2)Hospital General Universitario de Alicante- CIBERehd and Instituto de Investigación Sanitaria y Biomédica de Alicante ISABIAL, Gastroenterology Unit, Alicante, Spain;(3)Complexo Hospitalario Universitario de Santiago de Compostela, Gastroenterology Unit, Santiago de Compostela, Spain;(4)Hospital General Universitario de Valencia, Gastroenterology Unit, Valencia, Spain;(5)Hospital Universitario Puerta de Hierro Majadahonda, Gastroenterology Unit, Madrid, Spain;(6)Hospital Universitario y Politécnico La Fe and CIBEREHD, Gastroenterology Unit, Valencia, Spain;(7)Hospital Universitario Virgen de La Victoria, Gastroenterology Unit, Málaga, Spain;(8)Hospital Universitario de Cabueñes, Gastroenterology Unit, Gijón, Spain;(9)Hospital General Universitario de Cuidad Real, Gastroenterology Unit, Cuidad Real, Spain;(10)Hospital Universitario Fundación Alcorcón, Gastroenterology Unit, Madrid, Spain;(11)Hospital Universitario Reina Sofía, Gastroenterology Unit, Córdoba, Spain;(12)Hospital Universitario 12 de Octubre, Gastroenterology Unit, Madrid, Spain;(13)Hospital Universitario Marqués de Valdecilla e IDIVAL, Gastroenterology Unit, Santander, Spain;(14)Hospital Universitario Miguel Servet, Gastroenterology Unit, Zaragoza, Spain;(15)Hospital Universitario de Burgos, Gastroenterology Unit, Burgos, Spain;(16)Hospital Clínico Universitario Lozano Blesa and Fundación del Instituto de Investigación Sanitaria de Aragón IIS Aragón and CIBEREHD, Gastroenterology Unit, Zaragoza, Spain;(17)Instituto Biodonostia- Universidad del País Vasco UPV/EHU and CIBEREHD, Gastroenterology Unit, Guipúzcoa, Spain;(18)Hospital Álvaro Cunqueiro. Estrutura Organizativa de Xestión Integrada de Vigo, Gastroenterology Unit, Vigo, Spain;(19)Hospital General de Tomelloso. Instituto de Investigación Sanitaria Princesa IIS-IP and CIBEREHD, Gastroenterology Unit, Ciudad Real, Spain;(20)Hospital Universitario Infanta Sofía, Gastroenterology Unit, Madrid, Spain;(21)Hospital del Mar, Gastroenterology Unit, Barcelona, Spain;(22)Hospital Universitario La Paz e Instituto de Investigación Hospital Universitario La Paz IdiPAZ, Gastroenterology Unit, Madrid, Spain;(23)Hospital Universitario Clínico de Valencia, Gastroenterology Unit, Valencia, Spain;(24)Hospital Universitario San Jorge, Gastroenterology Unit, Huesca, Spain;(25)Hospital Universitario de Araba sede Txagorritxu y sede Santiago, Gastroenterology Unit, Álava, Spain;(26)Hospital Universitario Central de Asturias e Instituto de Investigación Sanitaria del Principado de Asturias ISPA, Gastroenterology Unit, Oviedo, Spain;(27)Hospital Universitario Vall d’Hebrón, Gastroenterology Unit, Barcelona, Spain;(28)Hospital Universitario de Bellvitge, Gastroenterology Unit, Barcelona, Spain;(29)Hospital Universitario Juan Ramón Jiménez, Gastroenterology Unit, Huelva, Spain; on behalf of DUMBO study group of GETECCU
Background
The safety of ustekinumab in pregnant patients with inflammatory bowel disease (IBD) and in their offspring has been barely studied.
Aims: Primary: To know the risk of serious adverse events (SAEs) in women exposed to ustekinumab during pregnancy and in their offspring. Secondary: To assess the risk of complications of the mothers and their offspring. To describe the patterns of use of ustekinumab during pregnancy in these patients
Methods
Patients with IBD exposed to ustekinumab during pregnancy from DUMBO registry of GETECCU were included. DUMBO is a prospective, observational and multicentre registry, which enrols pregnant women with IBD over 5 years in 70 centres in Spain. The registry was kicked off in September 2019. SAE definition was based on “Clinical Safety Data Management: Definitions and Standards for Expedited Reporting by European Medicines Agency”. Study protocol is summarized in figure 1.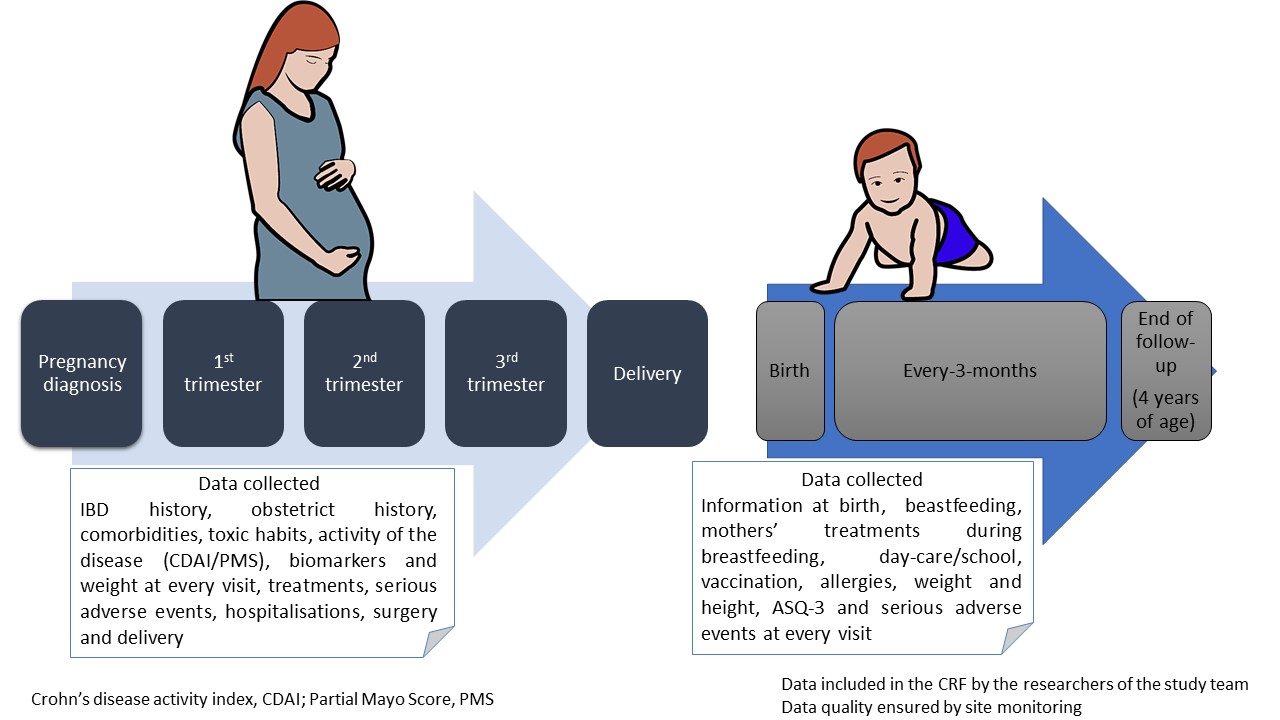
Results
49 pregnant patients have been exposed to ustekinumab during pregnancy so far (table 1). 
All pregnancies were singleton. Two patients were lost of follow-up (1st and 2nd trimester) but had uneventful pregnancies up-to last visit. There were 2 miscarriages (4%) at 1st trimester of gestation, 34 newborns and 11 pregnancies that were still on-going at the time of this analysis. All patients were on ustekinumab at conception (57% of them 90 mg/8 weeks). A total of 12 patients (24%) withdrawn ustekinumab during pregnancy: 1 (8%) due to disease flare, 1 (8%) underwent surgery due to intestinal obstruction, 2 (17%) due to patient’s choice (at 1st and 2nd trimester), and 8 (67%) due to clinicians’ decision (1 at 1st, 5 at 2nd and 2 at 3rd trimesters). No patient flared up after ustekinumab discontinuation. 10 (20%) patients had SAEs: 2 miscarriages, 1 intestinal infection, 1 subcorionic hematoma, 3 preterm birth, 1 intestinal obstruction and perforation (underwent surgery), 1 preeclapmsia, and 1 stoma obstruction. A total of 34 women gave birth after a median of 39 weeks gestation [interquartile range (IQR)=38-40], 3 (9%) preterm births, 55% by caesarean section (82% obstetric reasons and 18% perianal fistulae). Of the 34 newborns, 53% were female, median birth weight was 3,110 g (IQR=2,820-3,325), 3 (9%) low-birth weight, and 50% were breastfed exclusively. Median babies’ follow-up was 12 months (IQR=7-16). During follow-up, 3 children (9%) had severe infections (2 urinary infections, and 1 bronchiolitis by respiratory syncytial virus). In addition, 4 (13%) children were hospitalized: 1 cardiorespiratory arrest, 1 prematurity, 1 jaundice, and 1 vesicoureteral reflux
Conclusion
Ustekinumab seems to be safe during pregnancy in patients with IBD and their offspring.


