P548 Efficacy outcomes of placebo maintenance treatment in patients with moderate to severe Crohn’s disease who responded to placebo induction therapy: Post-hoc analysis of the Phase 3 ADVANCE, MOTIVATE, and FORTIFY Risankizumab Studies
Atreya, R.(1)*;Irving, P.(2);Fujiya, M.(3);Colombel, J.F.(4);Danese, S.(5);Peyrin-Biroulet, L.(6);Bessissow, T.(7);Panaccione, R.(8);D’Haens, G.(9);van Haaren, S.(10);Neimark, E.(11);Zambrano, J.(12);Zhang, Y.(13);Kligys, K.(10);Ferrante, M.(14);
(1)Friedrich-Alexander-Universität Erlangen-Nürnberg, Department of Medicine, Erlangen, Germany;(2)St Thomas' Hospital, IBD Centre- Department of Gastroenterology, London, United Kingdom;(3)Asahikawa Medical University, 3Department of Medicine- Division of Metabolism and Biosystemic Science- Gastroenterology and Hematology/Oncology, Asahikawa, Japan;(4)Icahn School of Medicine at Mount Sinai, Division of Gastroenterology, New York City, United States;(5)Humanitas Clinical and Research Center – IRCCS, IBD Center- Department of Gastroenterology, Rozzano, Italy;(6)University Hospital of Nancy-Brabois, Department of Gastroenterology, Vandœuvre-lès-Nancy, France;(7)McGill University Health Centre, Division of Gastroenterology, Montreal, Canada;(8)University of Calgary Cumming School of Medicine, Inflammatory Bowel Disease Unit, Calgary, Canada;(9)Amsterdam University Medical Centers- Academic Medical Centre, Inflammatory Bowel Disease Centre, Amsterdam, The Netherlands;(10)AbbVie, Global Medical Affairs, North Chicago, United States;(11)AbbVie, Clinical Development, North Chicago, United States;(12)AbbVie, US Medical Affairs, North Chicago, United States;(13)AbbVie, Statistics, North Chicago, United States;(14)University Hospitals Leuven- KU Leuven, Department of Gastroenterology and Hepatology, Leuven, Belgium;
Background
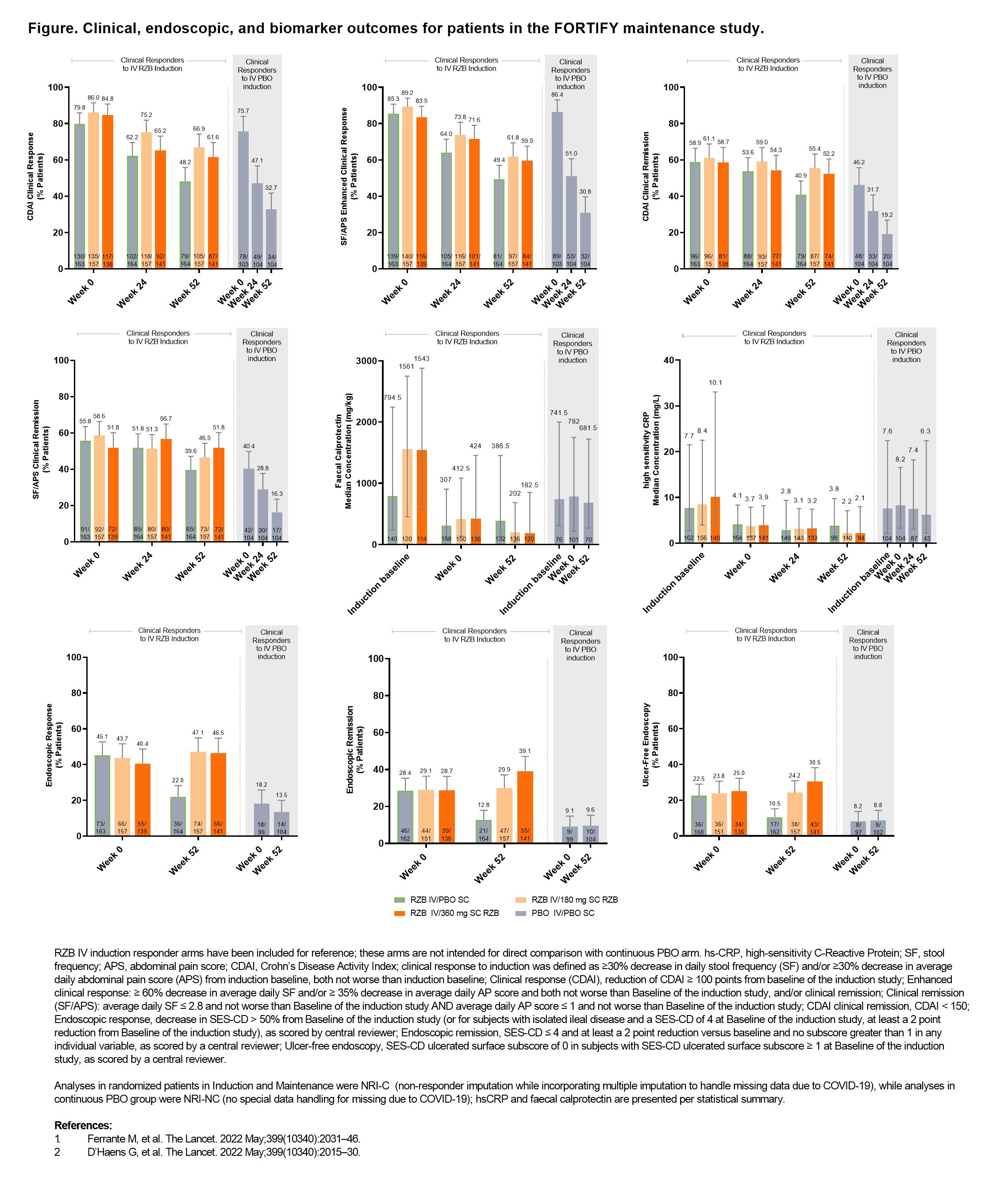
In the risankizumab (RZB) phase 3 clinical programme, Crohn’s disease (CD) patients who responded to RZB intravenous (IV) induction were re-randomised to RZB or placebo (PBO) (withdrawal PBO subcutaneous [SC] in maintenance (FORTIFY), while responders to PBO induction continued receiving PBO. Patients in the withdrawal (PBO SC) arm of FORTIFY who received IV RZB induction achieved high rates of clinical response/remission at Week (Wk) 52 likely due to the prolonged PK/PD effect of RZB and robust efficacy during induction.1 Here, we describe disease activity outcomes in non-randomised patients who received PBO during both induction and maintenance.
Methods
This post-hoc analysis included patients who received PBO IV in the induction study (ADVANCE or MOTIVATE), achieved clinical response (definition in Figure footnote) at Wk 12, and continued on PBO SC during FORTIFY.1,2 Subjective measures of disease activity (clinical remission [per CD Activity Index (CDAI)] and per stool frequency [SF] and abdominal pain score [APS], CDAI clinical response, and SF/APS enhanced clinical response), as well as objective measures of biomarkers (high sensitivity C-Reactive Protein and faecal calprotectin) levels and endoscopic outcomes (response, remission, and ulcer-free endoscopy), during FORTIFY were evaluated (endpoint definitions in Figure footnotes) at Wk 52. Biomarkers were additionally evaluated at induction baseline. For reference only, previously reported data from patients who were clinical responders to RZB IV induction and were re-randomised in FORTIFY are presented alongside data from the continuous PBO patients subgroup.1,2
Results
In the 104 patients who received PBO during both induction and maintenance, clinical response and remission rates decreased steadily during FORTIFY (Figure), while biomarker levels were relatively unchanged from induction baseline to FORTIFY Wk 52. In contrast, a slower rate of loss of response per clinical response/remission and lower biomarker levels over time were observed in RZB IV induction responders re-randomised to PBO in FORTIFY. Although already low, endoscopic response rates in the continuous PBO group decreased from FORTIFY Wk 0 to Wk 52, whereas endoscopic remission and ulcer-free endoscopy rates remained unchanged.
Conclusion
The symptomatic improvement achieved in patients clinically responding to PBO during induction was not maintained with continued PBO treatment in maintenance, unlike that observed in patients withdrawn from RZB, underscoring the durability of RZB induction response.1 Most objective measures of disease activity in patients receiving continuous PBO were relatively unchanged over time, with biomarkers remaining elevated and endoscopic rates of response low.