P550 Management of immunomodulators and biologic agents in pregnant patients with inflammatory bowel: results from the DUMBO registry of GETECCU
Chaparro, M.(1); G Donday, M.(1);Rubio, S.(2);Nuñez, A.(3);Calviño Suarez, C.(4);Madero, L.(5);Figueira, M.(6);Rivero, M.(7);Pérez Martínez, I.(8);Diz-Lois Palomares, M.T.(9);Huguet, J.M.(10);Marín Pedrosa, S.(11);Aguas, M.(12);Arroyo, M.(13);Ruiz-Cerulla, A.(14);Vázquez Morón, J.M.(15);Fernández-Clotet, A.(16);Guerra, I.(17);López Serrano, P.(18);Rodríguez-Lago, I.(19);Arias García, L.(20);Camargo Camero, R.(21);Casanova, M.J.(1);Martínez Montiel, P.(22);Sendra Rumbeu, P.(23);Suarez Ferrer, C.(24);Valldosera Gomis, G.(25);Armesto, R.(26);Bujanda, L.(27);Calvo Moya, M.(28);Hervías Cruz, D.(29);Robles Alonso, V.(30);de Jorge Turrión, M.Á.(31);Zúñiga de Mora-Figueroa, B.(32);Molina Arriero, G.(33);Acosta, D.(34);Brenes, Y.(34);Hermida, S.(34);Parra, P.(34);Gisbert, J.P.(1);
(1)Hospital Universitario de La Princesa- Instituto de Investigación Sanitaria Princesa IIS-IP and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas CIBERehd, Gastroenterology Unit, Madrid, Spain;(2)Hospital Universitario de Navarra, Gastroenterology Unit, Pamplona, Spain;(3)Hospital Universitario Virgen del Rocío, Gastroenterology Unit, Sevilla, Spain;(4)Complexo Hospitalario Universitario de Santiago de Compostela, Gastroenterology Unit, Santiago de Compostela, Spain;(5)Hospital General Universitario de Alicante, Gastroenterology Unit, Alicante, Spain;(6)Xerencia Xestión Integrada de Vigo- SERGAS. Grupo de Investigación de Patología Digestiva. Instituto de Investigación Sanitaria Galicia Sur IIS Galicia Sur. SERGAS UVIGO, Gastroenterology Unit, Vigo, Spain;(7)Hospital Universitario Marqués de Valdecilla e IDIVAL, Gastroenterology Unit, Santander, Spain;(8)Hospital Universitario Central de Asturias e Instituto de Investigación Sanitaria del Principado de Asturias ISPA, Gastroenterology Unit, Oviedo, Spain;(9)Complexo Hospitalario Universitario de A Coruña, Gastroenterology Unit, A Coruña, Spain;(10)Hospital General Universitario de Valencia, Gastroenterology Unit, Valencia, Spain;(11)Hospital Universitario Reina Sofía, Gastroenterology Unit, Córdoba, Spain;(12)Hospital Universitario y Politécnico La Fe and CIBEREHD, Gastroenterology Unit, Valencia, Spain;(13)Hospital Clínico Universitario Lozano Blesa and Fundación del Instituto de Investigación Sanitaria de Aragón IIS Aragón and CIBEREHD, Gastroenterology Unit, Zaragoza, Spain;(14)Hospital Universitario de Bellvitge, Gastroenterology Unit, Barcelona, Spain;(15)Hospital Universitario Juan Ramón Jimenez, Gastroenterology Unit, Huelva, Spain;(16)Hospital Universitario Clìnic i Provincial, Gastroenterology Unit, Barcelona, Spain;(17)Hospital Universitario de Fuenlabrada e Instituto de Investigación Hospital Universitario La Paz IdiPAZ, Gastroenterology Unit, Madrid, Spain;(18)Hospital Universitario Fundación Alcorcón, Gastroenterology Unit, Madrid, Spain;(19)Hospital Universitario de Galdakao- Instituto de Investigación Sanitaria Biocruces Bizkaia, Gastroenterology Unit, Galdakao, Spain;(20)Hospital Universitario de Burgos, Gastroenterology Unit, Burgos, Spain;(21)Hospital Universitario Virgen de La Victoria, Gastroenterology Unit, Málaga, Spain;(22)Hospital Universitario 12 de Octubre, Gastroenterology Unit, Madrid, Spain;(23)Hospital Universitario Son Espases, Gastroenterology Unit, Palma de Mallorca, Spain;(24)Hospital Universitario La Paz, Gastroenterology Unit, Madrid, Spain;(25)Hospital Universitario Joan XXIII, Gastroenterology Unit, Tarragona, Spain;(26)Complexo Hospitalario Universitario de Ourense, Gastroenterology Unit, Ourense, Spain;(27)Instituto Biodonostia- Universidad del País Vasco UPV/EHU and CIBEREHD, Gastroenterology Unit, Guipúzcoa, Spain;(28)Hospital Universitario Puerta de Hierro, Gastroenterology Unit, Majadahonda, Spain;(29)Hospital General Universitario de Cuidad Real, Gastroenterology Unit, Cuidad Real, Spain;(30)Hospital Vall d´Hebrón, Gastroenterology Unit, Barcelona, Spain;(31)Hospital Universitario de Cabueñes, Gastroenterology Unit, Gijón, Spain;(32)Hospital Clínico Universitario San Cecilio, Gastroenterology Unit, Granada, Spain;(33)Complejo Hospitalario Universitario de Ferrol, Gastroenterology Unit, A Coruña, Spain;(34)Hospital Universitario de La Princesa- IIS-IP and CIBEREHD, Clinical and Translational Research Unit. Inflammatory Bowel Disease Unit, Madrid, Spain; on behalf of DUMBO study group of GETECCU
Background
Most of the drugs approved for the treatment of inflammatory bowel disease (IBD) seem to be of low risk during pregnancy. However, clinicians’ attitude to IBD treatments during pregnancy in clinical practice has been barely studied. Aim: Primary: To describe the management of immunomodulators and biologic agents prescribed for IBD (initiation and withdrawal) in a prospective registry of clinical practice. Secondary aims: To know the main reasons for drug discontinuation; and to evaluate the impact of drug withdrawal on IBD activity during pregnancy.
Methods
Pregnant patients with IBD from DUMBO registry were included. DUMBO is a prospective, observational and multicentre registry, which enrols pregnant women with IBD over 5 years in 70 centres in Spain. The registry was kicked off in September 2019. Study protocol is summarized in figure 1.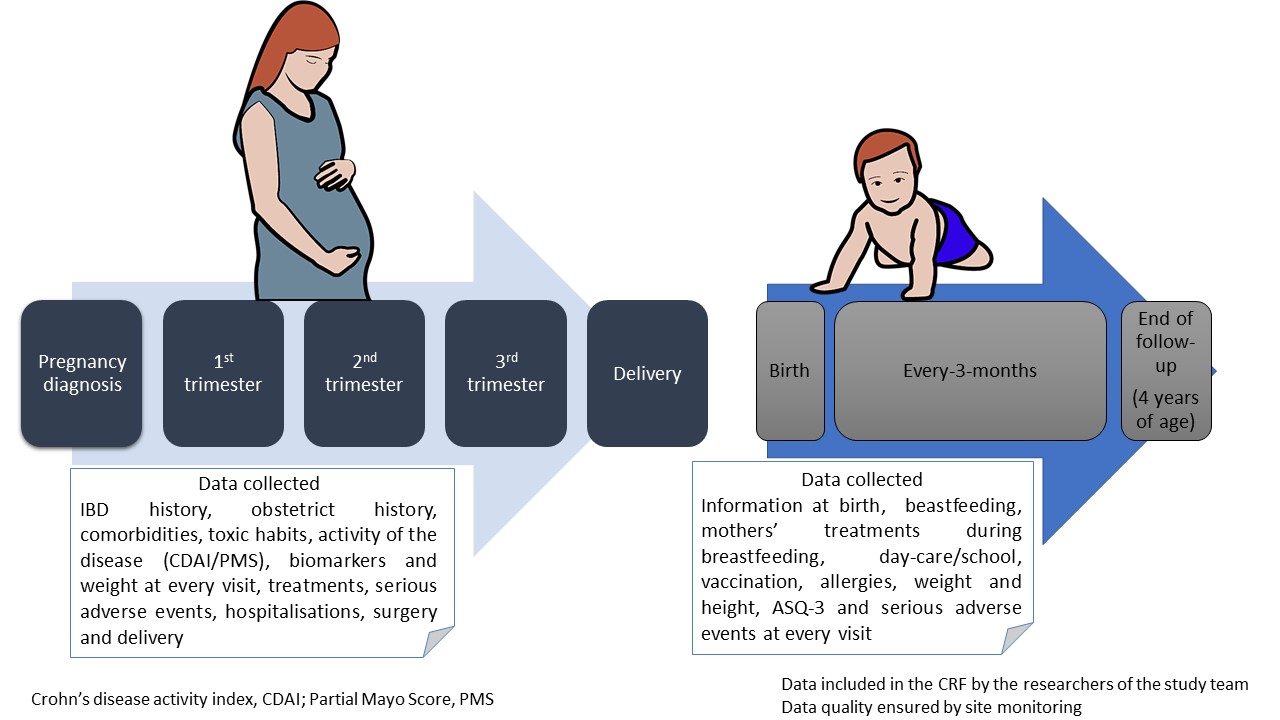
Results
So far, 580 pregnant women have been included in the registry; of them, 489 conceived at least 40 weeks before data extraction and were considered for the present analysis (Table 1). 
98% of the pregnancies were singleton, and 2% were twin pregnancies. There were 4% of miscarriages, and 1% of elective abortions. The majority of patients were in remission at conception and throughout pregnancy. Patients’ treatments during pregnancy are summarized in table 1; 20% of patients were exposed to thiopurines, 29% to biologics, and 10% to both biologics and thiopurines. No patient received methotrexate, cyclosporine, tacrolimus or tofacitinib during pregnancy. A minority of patients started a new drug during pregnancy: 3 patients (0.6%) started azathioprine, 2 (0.4%) adalimumab, 6 (1.2%) infliximab, and 1 (0.2%) ustekinumab. The proportion of patients who discontinued each drug, reasons for discontinuation, trimester of gestation at discontinuation and disease outcome, are summarized in table 2.

The proportion of patients who discontinued each drug among those with the standard or the intensified dose, are summarized in table 3. 
Of noted, biologic agents were discontinued in over 1/3 of patients, while thiopurines were maintained in most cases (78% of those who interrupted thiopurines were maintained on biologics). No patient flared-up after drug discontinuation (Table 2); all but one patient who interrupted the treatment, were in remission at baseline
Conclusion
Biologic agents (even anti-TNF agents) are withdrawn in a relatively high proportion of IBD patients during pregnancy, mainly due to clinicians’ decision. The risk of relapse after drug discontinuation is low, probably due to adequate control of the disease activity already at conception.


