P554 Extended induction response in patients treated with mirikizumab with moderately to severely active ulcerative colitis in the LUCENT trials
D'Haens, G.(1)*;Higgins, P.D.R.(2);Peyrin-Biroulet, L.(3);Sands, B.E.(4);Lee, S.(5);Moses, R.E.(6);Redondo, I.(7)*;Escobar, R.(8);Morris, N.(9);Kobayashi, T.(10);
(1)Amsterdam University Medical Centers, Inflammatory Bowel Disease Centre, Amsterdam, The Netherlands;(2)University of Michigan, Department of Internal Medicine, Michigan, United States;(3)University Hospital of Nancy, Gastroenterology, Nancy, France;(4)Icahn School of Medicine at Mount Sinai, Gastroenterology, New York, United States;(5)University of Washington Medical Center, Digestive Health Center, Washington, United States;(6)Eli Lilly and Company, Gastroenterology, Indianapolis, United States;(7)Eli Lilly Portugal, Produtos Farmacêuticos Lda., Lisbon, Portugal;(8)Eli Lilly Spain, Immunology, Alcobendas, Spain;(9)Eli Lilly and Company, Statistics, Indianapolis, United States;(10)Kitasato University -Kitasato Institute Hospital, Gastroenterology, Tokyo, Japan;
Background
The efficacy and safety of mirikizumab (miri) for moderately to severely active ulcerative colitis (UC) was demonstrated in the Phase 3 LUCENT trials (LUCENT-1 and -2; NCT03518086, NCT03524092).1,2 Some patients may improve more slowly and require a prolonged course of induction before achieving clinical response. Therefore, we evaluated clinical response in patients who had not responded by the end of the 12-week(W) induction period and who received extended induction treatment for an additional 12W.
Methods
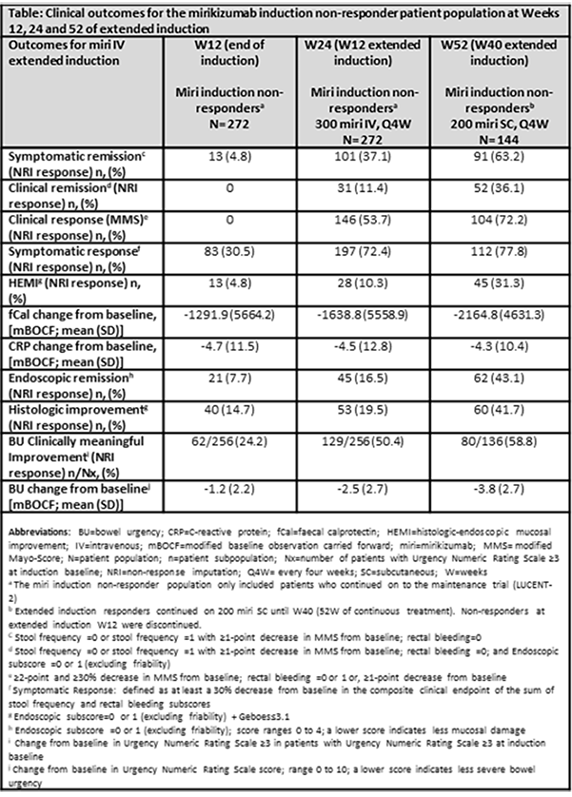
Patients not responding to intravenous (IV) administration of 300 mg miri every 4 weeks (Q4W) (n=272) at W12 (LUCENT-1), received extended 12-W induction treatment of open label 300 mg miri IV Q4W for 3 additional doses (LUCENT-2). Patients responding at W24 (n=144) of continuous IV treatment, entered open label maintenance and received 200 mg miri Q4W subcutaneously (SC) until W52. Key outcomes through 52W are reported (Definitions in Table).
Results
Among the 272 patients who failed to achieve a clinical response after 12W of induction treatment, 72.4% had a Mayo endoscopic subscore of 3, indicating severe disease. Of these patients, 146 (53.7%) achieved a clinical response and 31 (11.4%) achieved clinical remission at W24.
Of the 144 responders at 24W entering the open label maintenance study, 52 (36.1%) patients achieved clinical remission, 104 (72.2%) achieved clinical response, 62 (43.1%) achieved endoscopic remission and 60 (41.7%) patients achieved histologic improvement at W52. Furthermore, for these patients, there was a 3.8 (±2.7) point reduction from baseline in bowel urgency (BU) severity and 58.8% achieved clinically meaningful improvement in BU (Table). Among these patients, 38.3% demonstrated treatment-emergent adverse events (TEAEs). Most TEAEs were mild (21.4%), while only 5.4% were serious. Overall, there was a 3.2% of discontinuation due to TEAEs. No new safety signals or deaths were reported.
Conclusion
Among patients who did not initially respond to induction treatment with miri at 12W, 3 additional miri induction doses induced clinical response and remission in patients, highlighting the potential benefit of extended induction treatment with miri in those patients with more severe inflammation at baseline.
1.D’Haens et al., OP26: Efficacy and safety of mirikizumab as induction therapy in patients with moderately to severely active Ulcerative Colitis: Results from the Phase 3 LUCENT-1 study, Crohn's and Colitis, Vol. 16, Iss. Supplement_1, Jan. 2022, i028–i029
2.Dubinsky et al., 867e: Efficacy and safety of mirikizumab as maintenance therapy in patients with moderately to severely active Ulcerative Colitis: Results from the Phase 3 Lucent-2 study, Gastroenterology, Vol. 162, Iss. 7, S-1393-94