P558 Etrasimod for the treatment of ulcerative colitis: analysis of infection rates from the phase 3 ELEVATE UC 52 and ELEVATE UC 12 clinical trials
Regueiro, M.(1);Siegmund, B.(2)*;Yarur, A.(3);Steinwurz, F.(4);Gecse, K.B.(5);Goetsch, M.(6);Bhattacharjee, A.(7);Wu, J.(8);Green, J.(9);McDonnell, A.(10);Crosby, C.(11);Lazin, K.(12);Ferreira Branquinho, D.(13);Modesto, I.(14);Abreu, M.T.(15);
(1)Cleveland Clinic, Gastroenterology, Cleveland- Ohio, United States;(2)Medizinische Klinik für Gastroenterologie- Infektiologie und Rheumatologie- Charité - Universitätsmedizin Berlin, Gastroenterology, Berlin, Germany;(3)Cedars-Sinai Medical Center, Gastroenterology, Los Angeles- California, United States;(4)Hospital Israelita Albert Einstein, Gastroenterology, São Paulo, Brazil;(5)Amsterdam UMC, Gastroenterology, Amsterdam, The Netherlands;(6)Pfizer AG, Global Clinical Development, Zurich, Switzerland;(7)Pfizer Healthcare India Pvt. Ltd., Biostatistics, Chennai, India;(8)Pfizer Inc, Biostatistics, Groton- Connecticut, United States;(9)Pfizer Inc, Field Medical- Gastroenterology, Collegeville- Pennsylvania, United States;(10)Pfizer Ltd, Safety Risk, Walton Oaks- Scurry, United Kingdom;(11)Pfizer Inc, Translational Medicine, New York- New York, United States;(12)Pfizer AG, Clinical Development, Zurich, Switzerland;(13)Pfizer Inc, Global Medical Affairs- Gastroenterology, Lisbon, Portugal;(14)Pfizer Inc, Global Medical Affairs- Gastroenterology, Madrid, Spain;(15)University of Miami Miller School of Medicine, Gastroenterology, Miami- Florida, United States;
Background
Infections are an important safety concern in patients with IBD and may be due to its therapies, such as corticosteroids. Etrasimod is an investigational, once-daily, oral, selective sphingosine 1-phosphate receptor 1,4,5 (S1P1,4,5) modulator in development for the treatment of moderately to severely active ulcerative colitis (UC). The biologic effect of etrasimod leads to selective and reversible lymphocyte retention in lymph nodes with a decrease in peripheral lymphocyte count. We report the infection events from the phase 3 ELEVATE programme.
Methods
Infection events were evaluated in the pivotal UC pooled safety analyses set comprising two phase 3 studies: ELEVATE UC 52 (NCT03945188) and ELEVATE UC 12 (NCT03996369). Subjects (16-80 years) with moderately to severely active UC were randomised 2:1 to once-daily etrasimod 2 mg or placebo (PBO). We report the n (%) and exposure-adjusted incidence rate (EAIR) of infections including serious infections, severe infections, opportunistic infections (including tuberculosis), and herpes infections. Infections were considered adverse events of special interest (AESI) if they were severe (≥ CTCAE Grade 3), were opportunistic infections, or were herpes zoster or herpes simplex infections.
Results
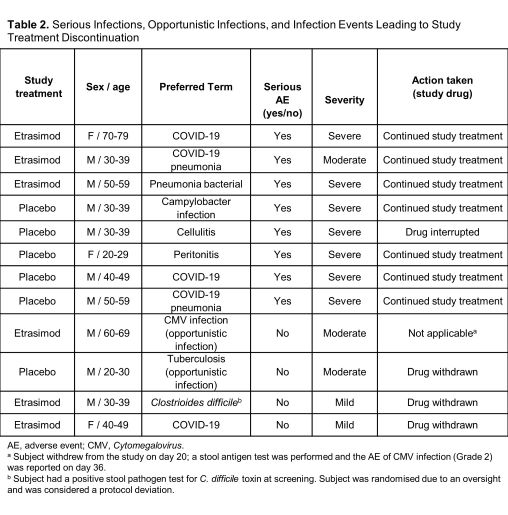
From the pooled ELEVATE UC 12 and ELEVATE UC 52 trials, 527 subjects received ≥1 dose of etrasimod 2 mg (265.6 subject-years of exposure) and 260 subjects were randomised to PBO (103.0 subject-years of exposure). Infections were similar between treatment groups (etrasimod: 99 [18.8%], EAIR=0.41; PBO: 46 [17.7%], EAIR=0.52). The most frequent infections in both groups were COVID-19, urinary tract infections, and nasopharyngitis (Table 1). Serious infections occurred in 3 (0.6%) subjects in the etrasimod arm (EAIR=0.01) and 5 (1.9%) in PBO arm (EAIR=0.05). Two cases of herpes zoster were reported in each treatment group (etrasimod: 0.4%, EAIR<0.01; PBO: 0.8%, EAIR=0.02); these were localised and nonserious. One opportunistic infection was reported in each arm (etrasimod: subject withdrew from the study on day 20, the AE of Cytomegalovirus infection [Grade 2] was reported on day 36; PBO: tuberculosis [Grade 2]). Overall, 3 cases of infection led to discontinuation: 2 in the etrasimod arm (both mild) and 1 in the PBO arm (Table 2). No subject with an absolute lymphocyte count <0.2×109/L subsequently reported a serious/severe or opportunistic infection. There were no deaths.

Conclusion
In these trials, etrasimod-treated subjects reported no increase in infections relative to PBO. Serious infections and herpes zoster were more commonly reported in the PBO-treated group. Longer-term follow-up data from the ongoing 5-year open-label extension will further characterize the etrasimod safety profile.


