P562 A Phase 1 Study Evaluating the Effect of Coadministration of Etrasimod on the Pharmacokinetics and Pharmacodynamics of a Monophasic Oral Contraceptive in Healthy Female Volunteers
Lee, C.(1);Tang , Y.(1);Villa-Caballero, L.(2);Liu , K.(3);Randle , A.(4);Grundy , J.(1);
(1)Arena Pharmaceuticals- Inc., Nonclinical Dev & Clinical Pharmacology, San Diego, United States;(2)Arena Pharmaceuticals- Inc., Clinical Development, San Diego, United States;(3)Arena Pharmaceuticals- Inc., Biostatistics & Data Mgmt, San Diego, United States;(4)Arena Pharmaceuticals- Inc., Clinical Operations, San Diego, United States;
Background
Etrasimod (ETR) is a once-daily (QD), oral, selective sphingosine 1-phosphate receptor modulator in clinical development for immune-mediated inflammatory disorders including inflammatory bowel disease (IBD) and atopic dermatitis. At diagnosis, ~50% of patients with IBD are <35 years old. Oral contraceptives (OC) are the most common birth control among women in the US. It’s important to ensure an IBD therapy does not impact the pharmacokinetic (PK)/pharmacodynamic (PD) performance of the OC. This study evaluated the effect of coadministration of ETR on the PK/PD performance of a monophasic OC.
Methods
This open-label Phase 1 study enrolled 21 premenopausal healthy female adults (18-40 years). On Days 1–21 of Period 1 (Days 1–28), participants received OC containing 30mcg ethinylestradiol (EE) and 50mcg levonorgestrel (LVG). On Day 23, participants initiated ETR 2mg QD such that steady-state concentrations were achieved by Day 28 and thereafter. On Days 29–49 of Period 2 (Days 29–50), the OC was coadministered with ETR 2mg QD. Samples for measuring the pharmacologically active components of the OC (EE and LVG) were collected over 24 hours on Days 21 and 49. Log-transformed primary PK parameters were calculated and compared using analysis of variance with treatment as fixed effect and participant as random effect. PD markers (follicle-stimulating hormone [FSH], luteinizing hormone [LH], estradiol, progesterone) were measured at baseline (Days 1 and 29) and throughout Periods 1 and 2. Largest ovarian follicle size measured by transvaginal ultrasound (TVUS), sex hormone–binding globulin (SHBG) levels, and Hoogland score were measured on Days 1 (before OC dosing), 11, 29 (before OC+ETR dosing), and 39. Safety and tolerability were also evaluated.
Results
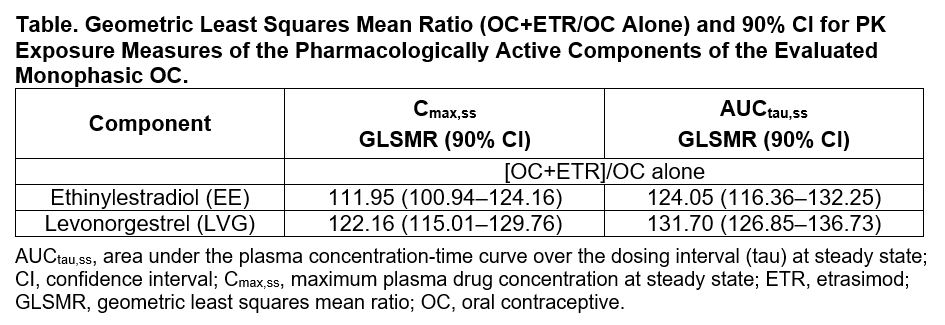
Only minor-to-mild increases in EE and LVG Cmax,ss and AUCtau,ss exposure measures were observed when the OC was coadministered with ETR vs when the OC was administered alone (Table). These changes were not considered clinically relevant. Markers of ovarian activity (FSH, LH, estradiol, progesterone, SHBG, TVUS/Hoogland score) demonstrated no clinically relevant impact of coadministration of ETR on the PD performance of the OC. All treatments were generally well tolerated. Most treatment-emergent adverse events (AEs) were mild or moderate; 2 participants had severe AEs. No abnormalities in laboratory parameters, vital signs, or 12-lead electrocardiogram assessments were noted.
Conclusion
The PK/PD performance of the monophasic OC was not altered to a clinically relevant extent; thus contraceptive efficacy was maintained during coadministration with ETR. ETR and the monophasic OC, administered alone or in combination, were generally well tolerated.