P562 Real world effectiveness and safety of ozanimod: One-year follow-up from a large tertiary center
Cohen, N.A.(1)*;Choi, D.(1);Garcia, N.(1);Choi, N.(1);Picker, E.(1);Krugliak-Cleveland, N.(1);Cohen, R.(1);Dalal, S.(1);Sakuraba, A.(1);Pekow, J.(1);Rubin, D.(1);
(1)University of Chicago Medicine, IBD Center- Section of Gastroenterology- Hepatology and Nutrition, Chicago, United States;
Background
Ozanimod is a first in class sphingosine-1-phosphate receptor modulator approved by the FDA for moderately-to-severely active ulcerative colitis (UC). Real world data describing use of ozanimod are limited. We provide one-year follow up results of our patient cohort treated with ozanimod.
Methods
This prospective, observational cohort study includes consecutive patients who initiated ozanimod at our center. We collected demographic, clinical, and laboratory data. Clinical disease activity was assessed using the Simple Clinical Colitis Activity Index (SCCAI). Clinical response was defined as a decrease in ≥3 points in SCCAI from baseline with only patients with clinically active disease included in the analysis, and clinical remission was defined as an SCCAI score of ≤ 2.
Results
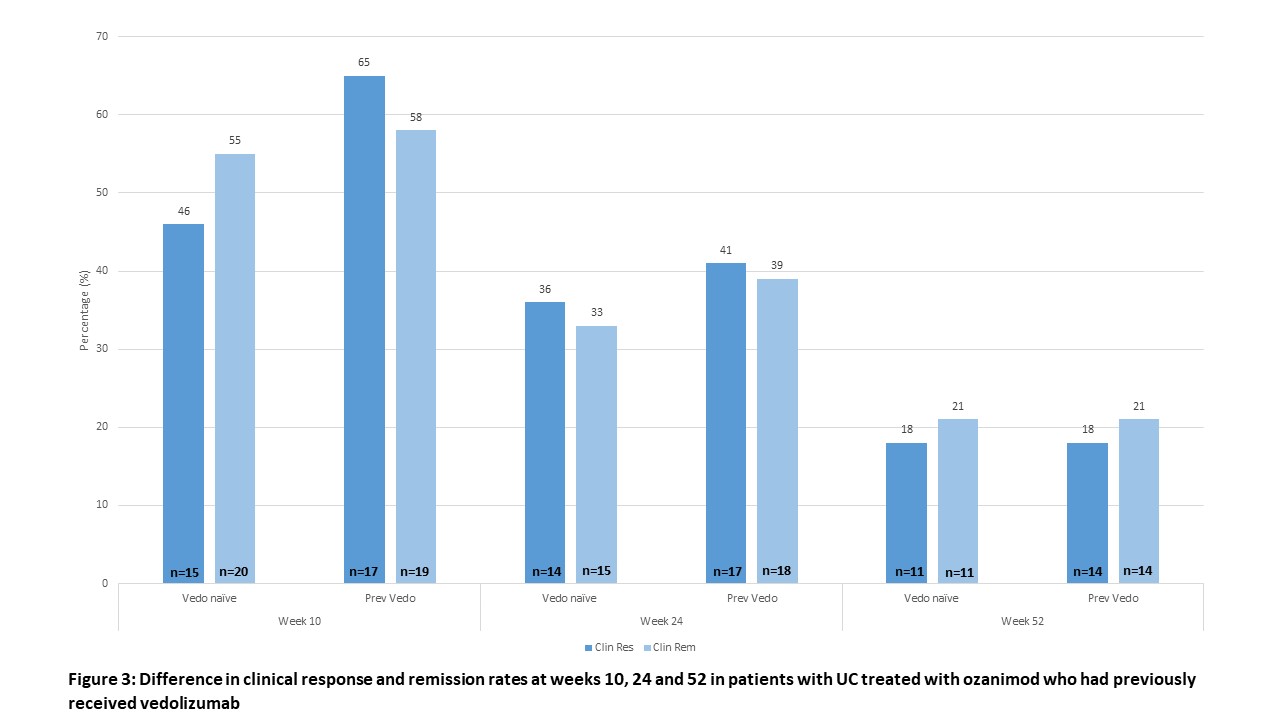
47 patients have initiated ozanimod therapy at our center: 45 patients with ulcerative colitis (UC), 1 patient with Crohn’s colitis and 1 patient with lymphocytic colitis. Only patients with UC were included in the effectiveness analysis. Demographics are in Table 1: in the UC cohort, the mean age was 40.6 ±16.6 years, mean disease duration was 9.6 ±9.2 years, 26 (58%) were male, 23 (51%) had extensive colitis, and 34 (76%) had previous advanced therapy exposure. 19 had baseline endoscopy with a mean Mayo endoscopic score of 2.6 ±0.5. Clinical response, remission, and corticosteroid free remission (CSFR) rates at weeks 2, 4, 10, 24, and 52 are in Figure 1. At week 10, follow-up was available for 41 patients: 18 patients (55%) achieved clinical response, 24 (59%) clinical remission, and 22 (54%) achieved CSFR. At week 52, follow-up was available for 30 patients: 5 (17%) achieved clinical response, 5 (17%) clinical remission, and 4 (13%) achieved CSFR. There were no significant differences between advanced therapy naïve and experienced patients, including when stratified by the number of previous advanced therapies or prior use of vedolizumab (Figures 2A+B and 3). Patients with a > 75% reduction in absolute lymphocytes had numerically greater induction clinical response and remission rates (80% vs 54%, p=0.4 and 75% vs 53%, p=0.4, respectively). 13 patients experienced adverse events (AEs): hypertension (n=2), transient nausea (n=2), fatigue (n=3), dry skin (n=2), transient liver enzyme elevation (n=2) and transient headache (n=3). Two AEs required discontinuation – hypertensive crisis in a patient with a prior dx of hypertension, and fatigue. There were no episodes of symptomatic bradycardia.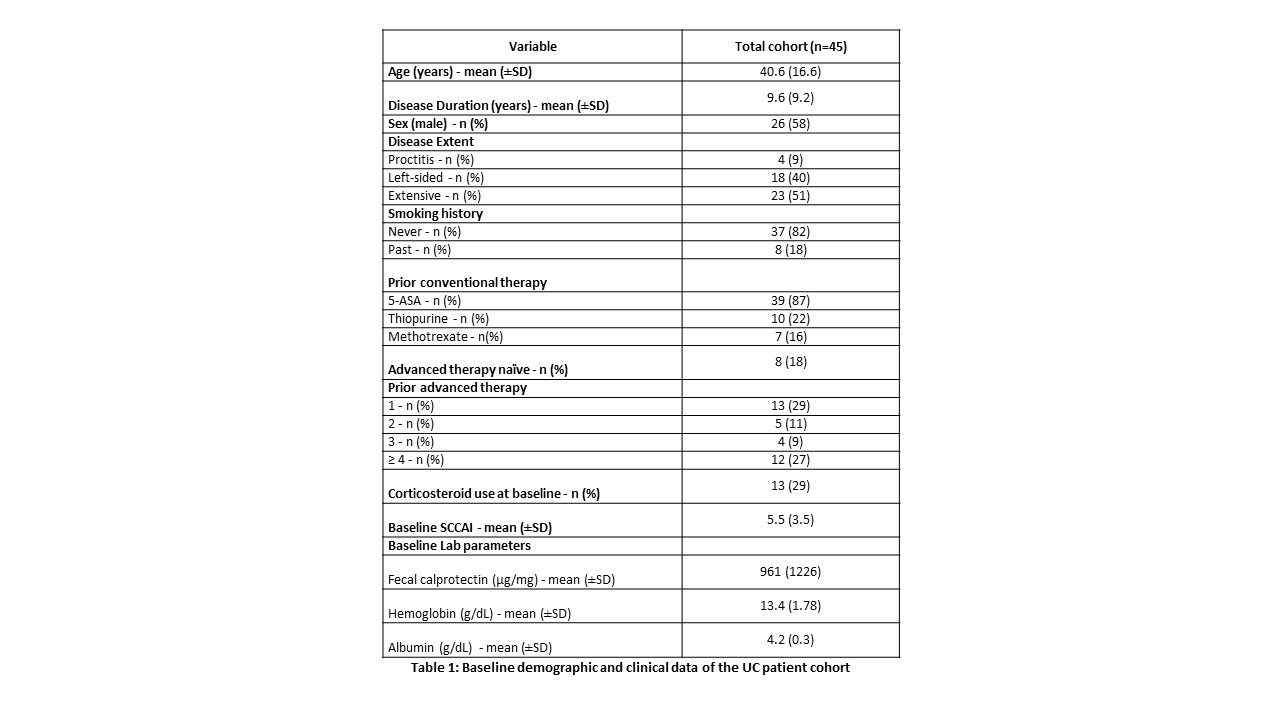


Conclusion
We present one-year real world follow-up of ozanimod in a largely treatment-refractory cohort of patients with UC, and describe modest one-year effectiveness and a safety profile that is not different from that described in the clinical trials.


