P564 vedolizumab versus infliximab and adalimumab in the real-world persistence in Ulcerative Colitis and Crohn’s disease – a meta-analysis
Yiu, T.H.(1)*;Leong, R.(2);Ko, Y.(2);Pudipeddi, A.(2);
(1)University of Sydney, Faculty of Medicine and Health, Sydney, Australia;(2)Concord Repatriation General Hospital, Gastroenterology and Liver Services, Sydney, Australia;
Background
In Ulcerative Colitis (UC), vedolizumab demonstrated increased efficacy over adalimumab in the VARSITY study. However, the real-world effectiveness of vedolizumab against adalimumab, infliximab and in UC and Crohn’s disease (CD) is not well-known. Drug persistence measures the duration of time from initiation to discontinuation of a therapy, is a surrogate marker that combines therapeutic efficacy, safety, and tolerability. This meta-analysis compared drug persistence of vedolizumab versus anti TNF alpha inhibitors (TNFi) adalimumab and infliximab in UC and CD.
Methods
Systematic review and meta-analysis were conducted with observational studies identified in electronic database – EMBASE and PubMed search from inception of 2017 to July 2022 and abstracts screened from recent gastroenterology conference – Digestive disease week 2022 San Diego. 6 observational studies evaluating persistence of vedolizumab versus TNFi (infliximab and adalimumab) among participants aged >18 years with diagnosis of IBD met inclusion criteria and were included.
Results
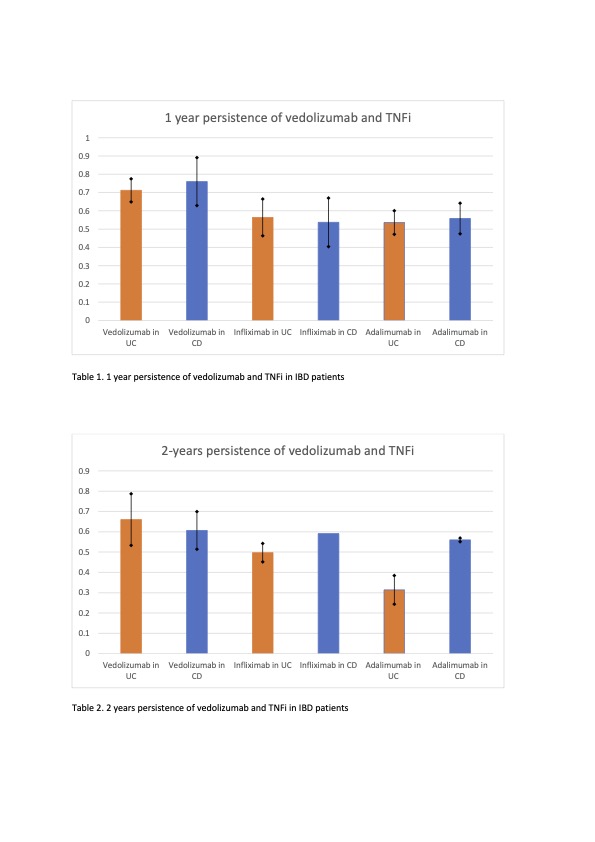
Overall, 1 year persistence of vedolizumab is 71.2% in UC and 76% in CD, significantly higher than infliximab (56.4% in UC, 53.7% in CD) and adalimumab (53.7% in UC, 55.6% in CD). (Table 1) Head-to-head comparison shows RR of 1.15 (95% CI of 1.12 – 1.19), favouring vedolizumab.
2-years persistence was pooled from 4 studies. Vedolizumab had a 2-years persistence of 66% in UC and 61% in CD, which also shows superiority to both infliximab (49.7% for UC, 59.1% for CD) and adalimumab (31.4% for UC and 56% for CD). (Table 2) Meta-analysis with head-to-head comparison continue to show a statistically significant difference in 2 years persistence of vedolizumab versus TNFi (RR of 1.12, 95% CI 1.01 – 1.25).
For UC patients, vedolizumab shows superiority over both adalimumab and infliximab with a RR of 1.41 (95% CI 1.14 – 1.74) and 1.15 (95% CI 1.06 – 1.25) respectively and RR of 1.23 (CI 1.14 – 1.33) when adalimumab and infliximab combined.
For CD, vedolizumab has a slightly higher 1-year persistence over TNFi combined (RR 1.10 95% CI 1.02 – 1.19) but fails to show any statistically significant difference from adalimumab (RR 1.07 95% CI 0.86 – 1.32) and infliximab (RR 0.55 95% CI 0.07 – 4.35) individually.
Vedolizumab has a higher 1-year persistence in bio-naïve subgroup (RR 1.14 95% CI 1.07 – 1.22) but fails to show statistically significant advantage in bio-experienced group (RR 1.04 95% CI 0.80 – 1.35) when compare with TNFi.
Conclusion
Vedolizumab achieved a higher overall 1- and 2-years persistence compared to adalimumab and infliximab in both CD and UC but mainly in bio-naïve subjects. The benefit of vedolizumab over TNFi was more evident in UC than CD.