P566 Humoral immune response to mRNA COVID-19 vaccine in Puerto Ricans with IBD does not differ between class of biologics
López Marte, P.(1);Ramos-Tollinchi, L.(1);Rodriguez-Martinó, E.(2);Medina-Prieto, R.(2);Ojeda, S.(3);Santana-Bagur, J.(4);Pantoja, P.(5);Sariol, C.A.(5);Torres, E.A.(2);
(1)University of Puerto Rico-School of Medicine, Gastroenterology Research, San Juan, Puerto Rico;(2)University of Puerto Rico-School of Medicine, Department of Medicine- Division of Gastroenterology, San Juan, Puerto Rico;(3)University of Puerto Rico-School of Medicine, Department of Medicine, San Juan, Puerto Rico;(4)University of Puerto Rico-School of Medicine, Department of Medicine- Division of Infectious Diseases, San Juan, Puerto Rico;(5)University of Puerto Rico-School of Medicine, Department of Microbiology, San Juan, Puerto Rico;
Background
The COVID-19 vaccine trials did not include subjects with IBD or immunosuppression, limiting the effectiveness data of the vaccine in this population. The impact of biologics with different mechanisms of action on antibody concentrations and neutralizing capacity produced by the vaccine may alter prevention strategies. We aim to describe a comparison of the humoral response to COVID-19 vaccine in patients with IBD on biologics between different therapies and a control group.
Methods
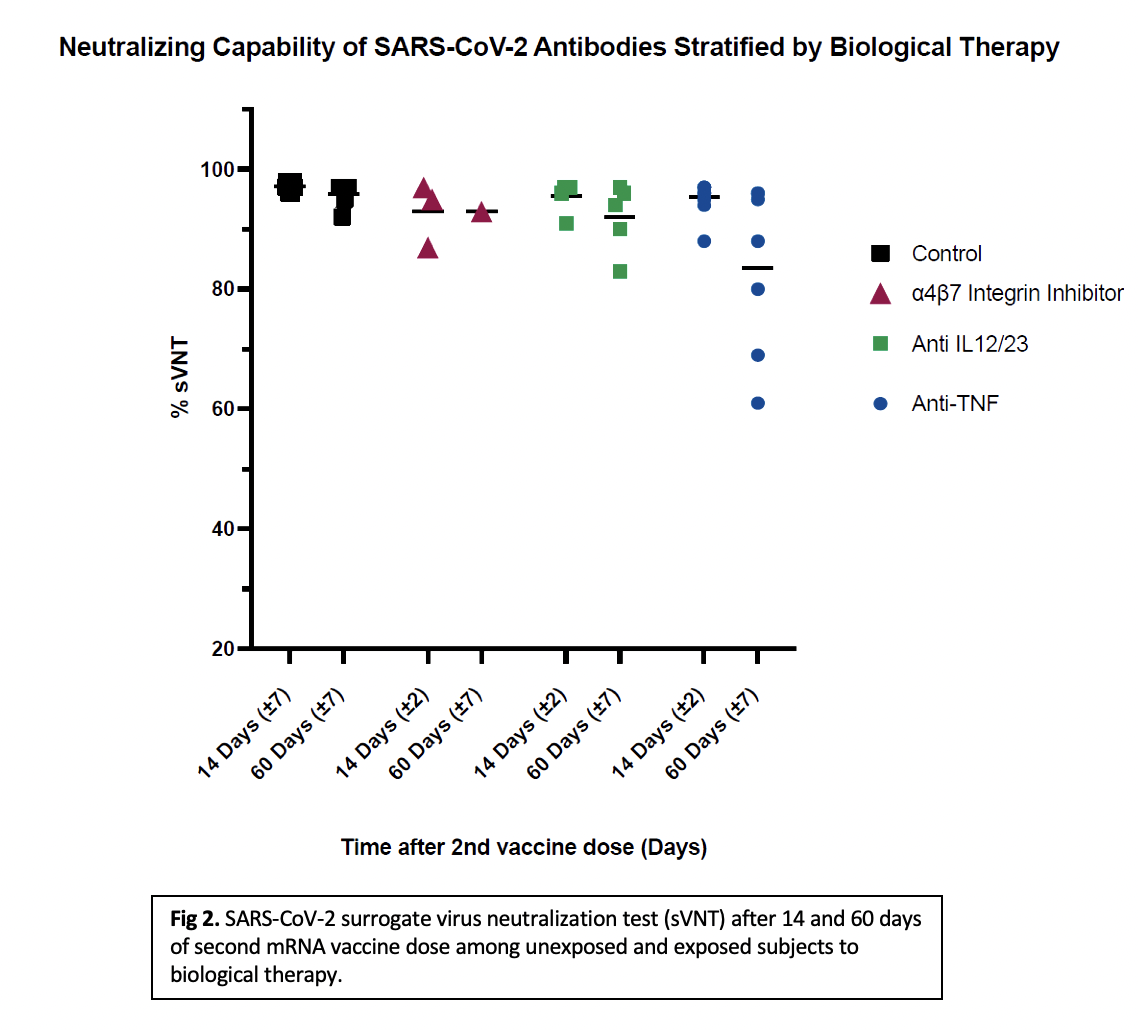
Patients ≥21 years of age with Crohn’s disease (CD) and ulcerative colitis (UC) on biologic therapy were recruited. Blood samples were collected at 14 ± 2 days (Cohort 1) and 60 ±7 days (Cohort 2) after receiving a 2nd dose of mRNA COVID-19 vaccine [BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna). Anti-spike protein receptor binding domain IgG levels and neutralizing capability using SARS-CoV-2 surrogate virus neutralization test (sVNT%) were measured. A result >30% is positive for effective viral inhibition.
Results
Results were stratified by mechanism of action of the drug and compared with a healthy control group. Comparisons were evaluated by Kruskal-Walls test and Dunns Pairwise/Bonferroni. The study is approved by the MSC IRB.32 subjects exposed to biological therapy divided into 2 cohorts (14-days, n=19/60-days, n=13) and 18 samples of healthy controls (14-days, n=12/60-days, n=6) are reported. Of the IBD subjects, 81% (26/32) had CD, 56% were males (18/32) and mean age was 39. All subjects were receiving biological monotherapy, one subject was also on azathioprine. All groups developed detectable antibody levels and >60% neutralizing antibody detection after 14 and 60 days of the second vaccine dose. IgG levels at 14 days (p<0.001) and sVNT% at 14- and 60-days post second vaccine dose (p=0.007, 0.024) were significantly different between subjects vs. controls. When stratified by mechanism of action, there was a significant difference between each biologic vs. control (p<0.001), but no difference between biologic classes. Initial analysis for the sVNT% at 14 days showed statistical significance (p=0.046). Post-hoc analysis showed no statistical significance in the individual comparisons. No significant differences were found between each therapy vs. control for IgG levels nor sVNT% at 60-days (p=0.257, 0.113).


Conclusion
Our study shows that IBD patients on biological therapy who receive a COVID-19 vaccine develop a lower but effective humoral response up to 60 days after the second dose when compared to healthy individuals. No difference was found between class of biologics. A larger sample is needed.


