P574 Exploring treatment of Crohn’s disease and ulcerative colitis in the Middle East and Northern Africa: An analysis of the HARIR observational cohort study
Hamed, W.(1);Salem, O.E.(2);Alharbi, O.(3);,4;Sharaf, M.(5);Taylor, C.(6);Sherief, A.(7);Besar, A.(6);
(1)Ain Shams University Hospital, Department of Tropical Medicine, Cairo, Egypt;(2)Osama Ebada GI Center, Department of Internal Medicine, Alexandria, Egypt;(3)King Saud University, College of Medicine, Riyadh, Saudi Arabia;(4)King Khalid University Hospital, College of Medicine, Riyadh, Saudi Arabia;(5)Janssen, Medical Affairs, Jeddah, Saudi Arabia;(6)Janssen, Medical Affairs, Dubai, United Arab Emirates;(7)Janssen, Medical Affairs, Cairo, Egypt
Background
This analysis of the HARIR study explored disease characteristics, treatment and outcomes of patients (pts) with diseases including Crohn’s disease (CD) or ulcerative colitis (UC) treated with biologics in clinical practice where data are limited: North Africa, the Middle East and Western Asia.
Methods
HARIR was a prospective, observational, multicentre, cohort, phase 4 study (NCT03006198). This analysis included adult pts with CD or UC who were starting infliximab, golimumab or ustekinumab (study period: March 2016–December 2018; terminated early). Pts needed to be previously untreated with study drugs or received ≤2 biologics prior to enrolment. Treatment was at physician’s discretion. Pts were followed for 1 year or until study withdrawal (amended from 2 years); pts who stopped the study due to study termination were considered to have completed the study. For CD, the main efficacy outcomes were Crohn’s Disease Activity Index (CDAI) treatment response, remission and change from baseline of inflammatory Bowel Disease Questionnaire (IBDQ). Adverse events (AEs) and serious AEs (SAEs) were recorded.
Results
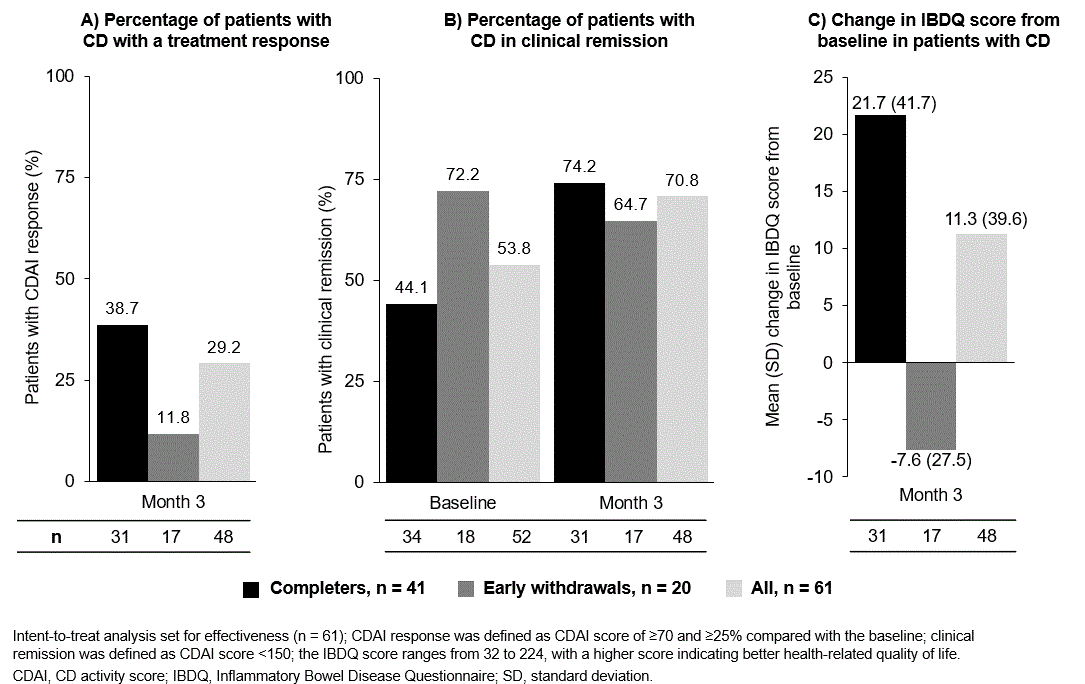
A total of 86 pts with CD or UD from Algeria, Egypt, Kuwait, Qatar and Saudi Arabia were enrolled; 56 pts completed the study. Target enrolment was achieved for CD but was 1 pt short for UC. Mean (SD) age was 32.7 (11.5) years for CD and 29.6 (9.4) years for UC. Most pts with CD and UC had not previously received biologics (CD: 95.2%, 59/62; UC: 66.7%, 16/24). All pts with CD and UC received infliximab. Investigators noted lack of access to study drug and concomitant treatments as limiting factors for enrolment. Immunosuppressant use was low; methotrexate was used by 1 pt with UC prior to study treatment and azathioprine or mycophenolate mofetil were used by 19.4% (12/62) of pts with CD and 24.0% (6/25) of pts with UC during the study. At Month 3, 29.2% (14/48) of pts with CD had a positive treatment response (CDAI score ≥70 to ≥25% versus baseline; Figure). The percentage of pts with CD in clinical remission was 53.8% (28/52) at baseline and 70.8% (34/48) at Month 3 (Figure). Mean (SD) IBDQ score increased by 11.3 (39.6%) points from baseline to Month 3 (Figure). Overall, pt numbers were too low to analyse outcomes for CD after Month 3 and over the study duration for UC. In CD and UC, respectively, AEs were reported by 25.8% (16/62) and 37.5% (9/24) of pts and SAEs were reported by 12.9% (8/62) and 16.7% (4/24) of pts.
Conclusion
Infliximab treatment was well tolerated, and a moderate clinical response was observed for this Middle Eastern and Northern African population. Limited accessibility to biologics and concomitant treatments in these countries restricted conduct of the study.