P577 A Phase 1b Study to Evaluate Safety, Tolerability, Pharmacokinetics and Clinical Efficacy of the Nucleotide-binding oligomerization domain, Leucine rich Repeat containing X1 (NLRX1) agonist NX-13 in Ulcerative Colitis
Peyrin-Biroulet, L.(1)*;Danese, S.(2);Colombel, J.F.(3);Rieder, F.(4);Yarur, A.(5);Verstockt, B.(6);Lichtiger, S.(7);Mosig, R.(8);Cataldi, F.(7);Feagan, B.(9);
(1)Nancy University Hospital- University of Lorraine, Department of Gastroenterology and Inserm NGERE U1256, Vandoeuvre-lès-Nancy, France;(2)IRCCS San Raffaele Hospital and Vita-Salute San Raffaele University, Department of Gastroenterology and Digestive Endoscopy, Milan, Italy;(3)Icahn School of Medicine at Mount Sinai, The Dr. Henry D. Janowitz Division of Gastroenterology, New York, United States;(4)Cleveland Clinic, Department of Inflammation and Immunity- Lerner Research Institute- Department of Gastroenterology- Hepatology and Nutrition- Digestive Disease Institute, Cleveland, United States;(5)Cedars Sinai Medical Center, Division of Gastroenterology and Hepatology- Center for Inflammatory Bowel Diseases, Los Angeles, United States;(6)University Hospitals Leuven- KU Leuven, Department of Gastroenterology and Hepatology- Department of Chronic Diseases and Metabolism, Leuven, Belgium;(7)Landos Biopharma- Inc, Clinical, Blacksburg, United States;(8)Landos Biopharma- Inc, Corporate Development, Blacksburg, United States;(9)Western University, Division of Gastroenterology- Department of Medicine, London, Canada;
Background
NLRX1 is a mitochondrial membrane protein whose activation reduces oxidative stress and decreases effector cell differentiation and cytokine release. NX-13 is a first-in-class, orally active, gut selective NLRX1 agonist with low systemic exposure. In preclinical studies of IBD, NX-13 effectively reduced inflammatory responses and disease severity. A phase 1a study (healthy subjects) showed NX-13 to be well tolerated with low systemic drug exposure suggesting a gut-selective drug delivery. We report the results of a phase 1b study in patients with Ulcerative colitis (UC) on the safety, target engagement and clinical efficacy of NX-13.
Methods
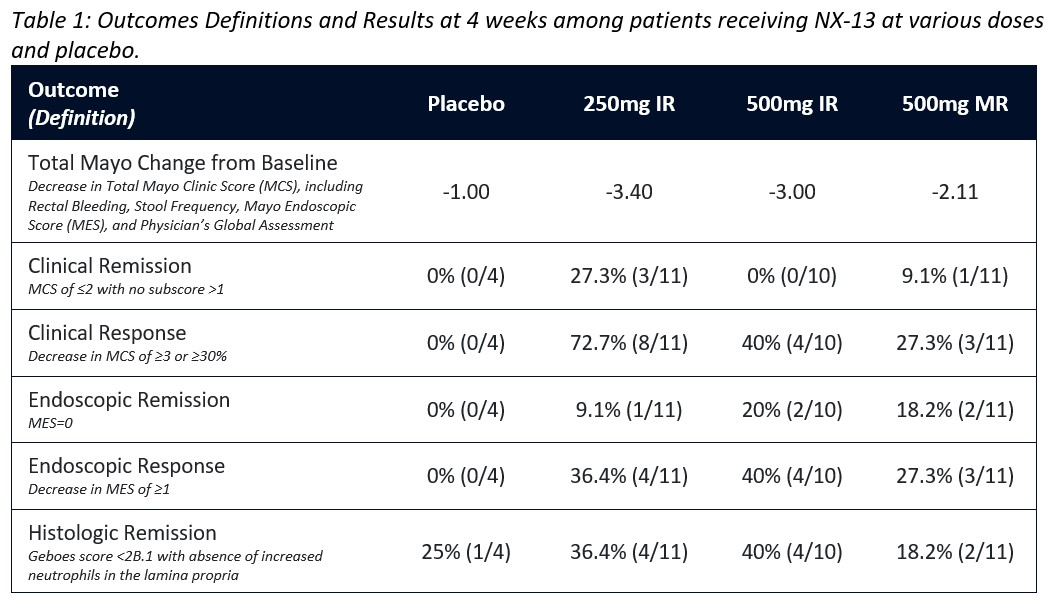
In this double-blind trial, 36 patients with active UC (Total Mayo Score [MCS] 4-10; Mayo endoscopic subscore [MES] 2-3) were randomly assigned to NX-13 250mg Immediate Release (IR), 500mg IR, 500mg Modified Release (MR) or Placebo to be taken QD for 4 weeks (safety visit at week 5). Biologic exposed patients, stable 5’ASAs dose and corticosteroids (oral ≤20mg/day prednisone or equivalent) were permitted. IV or topical corticosteroids use was prohibited. Primary endpoints were safety and pharmacokinetic (PK) analysis. The study was not powered for clinical efficacy, however MCS, histopathologic Geboes score, and NLRX1, cytokine, and gene expression were obtained at baseline and day 28 in exploratory analyses (Table 1).
Results
During the 5-week observation period, no serious adverse events (AEs) were reported. All AEs were classified as mild or moderate and were most common in the 500mg MR group. One patient was withdrawn before week 5 due to a flare of UC. IR dose plasma PK levels peaked at 1hour, while the MR dose was absorbed later and maintained a low exposure longer. Qualitative immunohistochemical analysis showed target engagement as indicated by increase of NLRX1 at all doses. Symptomatic efficacy was seen in 8/11 patients in the 250mg group with a PRO (RB+SF) score of 0 at week 4. The decrease in MCS was 2.1-3.4 in the NX-13 arms vs 1 point with placebo (Fig 1A) while clinical response rates ranged from 27-72% amongst the active arms with no placebo responders, though no dose-response relationship was observed (Fig 1B). Endoscopic response ranged from 27-40% (Fig 1C), with 5/32 dosed patients being in endoscopic remission. Histologic remission largely paralleled endoscopic response and cytokine and mRNA levels support target engagement.
Conclusion
In patients with active UC, NX-13 was well-tolerated and target engagement was achieved. The exploratory efficacy endpoints indicated rapid clinical, endoscopic, and histologic improvement at week 4, relative to placebo. This novel mechanism of action will be further evaluated in a phase 2 study.


