P578 Non-medical switch between adalimumab biosimilars and from the originator adalimumab to biosimilars in inflammatory bowel disease patients – a multicentre study on efficacy and drug sustainability
Lontai, L.(1);Gonczi, L.(1);Balogh, F.(1);Komlodi, N.(1);Resal, T.(2);Farkas, K.(2);Molnar, T.(2);Golovics, P.(3);Schafer, E.(3);Szamosi, T.(3);Miheller, P.(4);Ilias, A.(1);LakatosPhD, P.L.(1,5);
(1)Semmelweis University, Department of Medicine and Oncology, Budapest, Hungary;(2)University of Szeged, Department of Medicine, Szeged, Hungary;(3)Hungarian Defence Forces - Medical Centre, Department of Gastroenterology, Budapest, Hungary;(4)Semmelweis University, Department of Surgery and Interventional Gastroenterology, Budapest, Hungary;(5)Mcgill University Health Center, IBD Centre, Montréal, Canada;
Background
The use of biosimilar adalimumab (ADA) is effective and safe in inflammatory bowel disease (IBD), although clinical data on switching between ADA biosimilars is still rare. At the end of 2020, a non-medical switch to biosimilar ADA became mandatory in Hungary due to reimbursement policy changes of the National Health Insurance Fund of Hungary (NEAK). The aim of the present study was to evaluate short- and medium term clinical efficacy, drug sustainability and safety comparing non-medical switches from the originator to biosimilar ADA, and between ADA biosimilars.
Methods
246 consecutive patients on maintenance ADA therapy (n=181 Crohn’s disease [CD] and n=65 ulcerative colitis [UC], male/female: 44%/56%, median disease duration: 10years(y) (IQR: 10-16)) were included from 4 IBD centers between September 2019 and December 2020. Data on clinical efficacy, using Crohn’s Disease Activity Index (CDAI) and partial Mayo Score (pMayo), laboratory parameters (C-reactive protein – CRP) and adverse events were collected at 8-12 weeks prior switch, at baseline, and 8-12 weeks, 20-24 weeks after switch. Drug sustainability following the switch was evaluated after a median of 41 weeks (IQR: 35-42) follow-up time.
Results
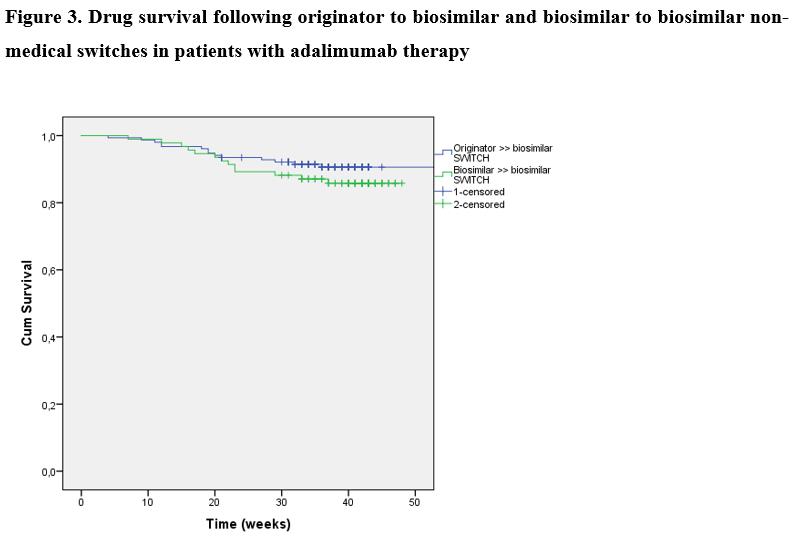
A total of 246 IBD patients (n=153 patients [115CD/38UC, median age: 38y(IQR: 27-45)] and n=93 patients [66CD/27UC, 32y(IQR: 26-40.5)] underwent a non-medical switch from the originator to a biosimilar, and biosimilar to biosimilar. Clinical disease activity based on CDAI and pMayo scores are presented in Figures 1 and 2. No significant difference was found in the proportion of patients in clinical remission at week 8-12 prior switch / switch / week 8-12 and week 20-24 in either patients switched from originator to biosimilar (86.8% / 88.2% / 86.0% / 85.0%; p=0.87 among groups) or biosimilar to biosimilar (72.0% / 77.4% / 84.9% / 77.6%; p=0.21). 89.2% and 83.1% of patients who were in clinical remission at switch/baseline sustained clinical remission up to week 20-24 in the first and second cohorts. Mean CRP levels were also unchanged during follow-up in both cohorts (p=0.71 and p=0.94). Drug survival was similar between originator to biosimilar and biosimilar to biosimilar switch cohorts, with a probability of 90.6% (SE: 2.4) and 85.8% (SE:3.7) to stay on drug after 40 weeks (log-rank: p=0.271). Figure 3. Two cases of skin reactions were registered as adverse events, one leading to treatment discontinuation.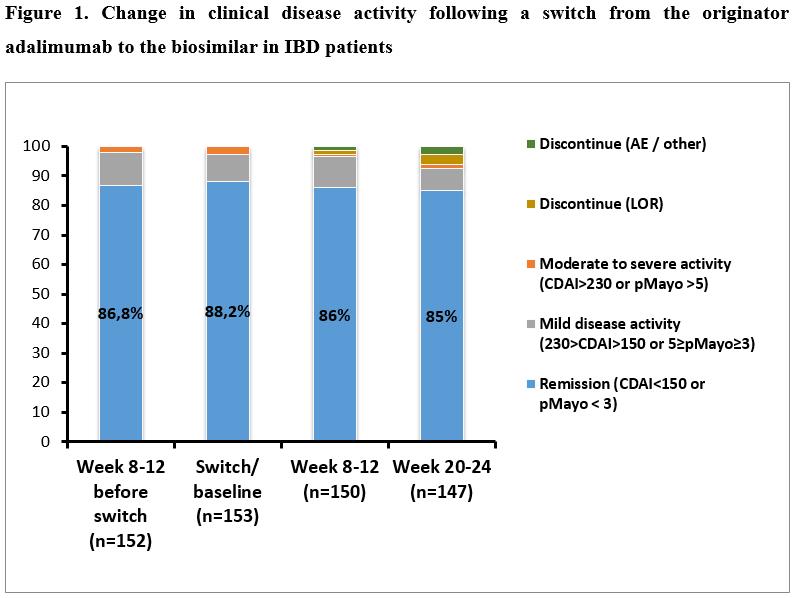

Conclusion
Clinical remission was sustained following non-medical switch from originator or biosimilar adalimumab to a biosimilar in IBD patients. Medium-term drug sustainability following the switch was high, and comparable between patients with an originator to biosimilar and a biosimilar to biosimilar switch.


