P580 Upadacitinib is effective and safe for Ulcerative Colitis: prospective real-world experience
Friedberg, S.(1)*;Choi, D.(1);Hunold, T.(1);Choi, N.K.(1);Garcia, N.M.(1);Picker, E.A.(1);Cohen, N.A.(1);Cohen, R.D.(1);Dalal, S.R.(1);Pekow, J.(1);Sakuraba, A.(1);Krugliak Cleveland, N.(1);Rubin, D.T.(1);
(1)University of Chicago, Inflammatory Bowel Disease Center, Chicago, United States;
Background
Upadacitinib (upa) is a novel selective JAK-1 inhibitor that has demonstrated efficacy in the treatment of moderate to severe ulcerative colitis (UC) and Crohn’s disease (CD) and has received regulatory approval for UC. We report a large prospective real-world experience with upa in patients with UC.
Methods
We performed a prospective analysis of clinical outcomes during upa therapy in patients with UC using pre-determined follow-up intervals at weeks 0, 2, 4, and 8 as part of a formalized treatment protocol. We used the Simplified Clinical Colitis Activity Index (SCCAI), C-reactive protein (CRP) and fecal calprotectin (FCP). Clinically active disease was defined as SCCAI ≥ 3. Clinical response was defined as a decrease of ≥ 3 points from baseline SCCAI. Clinical remission was defined as SCAAI < 3. Corticosteroid free clinical remission (CSFR) was defined as those in clinical remission, not taking systemic corticosteroid at the specific time point. CRP normalization was defined as baseline elevated and then within the normal range for the lab used (Quest Diagnostics (Secaucus, New Jersey) ≤8 mg/L or UChicago Medicine ≤5 mg/L). FCP response and remission was assessed at three different cutoffs: less than 250 μg/g, 150 μg/g, and 50μg/g. Adverse events were recorded as treatment-related adverse events (AE) and serious adverse events (SAE).
Results
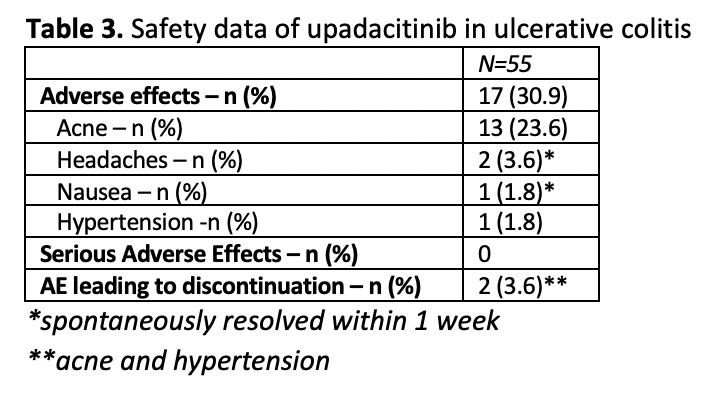
55 patients with UC received ≥4 weeks of upa (Table 1). 100% had previously received anti-TNF therapy and 81.8% had received ≥2 advanced therapies (biologic, small molecule, calcineurin-inhibitor). At 4 and 8 weeks of treatment, 18/22 (81.8%) and 19/21 (90.5%) achieved clinical response and 17/22 (77.3%) and 17/21 (81.0%) achieved clinical remission, respectively (Table 2). Of those who were on steroids at baseline, 100% (7 of 7) achieved CSFR. Results were seen as early as week 2 with clinical remission rates of 36.0%. Mean SCCAI decreased from 5.3 (SD 3.6) at week 0 to 1.71 (SD 3.96) at week 8 (p = <0.0001, Figure 1). Mean FCP decreased from 1028.5 (SD 1291) μg/g at week 0 to 530.2 (SD 715.1) μg/g (p = 0.0881). Of those with FCP >150 μg/g at baseline, 33.3% (2/6) achieved FCP <150 μg/g by week 8 (Table 2). Mean CRP (mg/L) decreased from 5.1 (SD 5.0) mg/L at week 0 to 2.5 (SD 3.3) mg/L at week 8. Of those with CRP >5 at week 0, 60% (3/5) achieved CRP <5 by week 8 (Table 2). 30.9% of patients had an AE after starting upa (Table 3). The most common of these was acne (23.6%). Two AEs led to discontinuation. There were no SAEs.


Conclusion
In this large prospective real-world experience in medically-resistant patients with UC, we describe that upadacitinib is rapidly effective and safe, with remission rates that exceed those seen in the pivotal trial.


