P588 Interim results from the randomised VERDICT trial to determine the optimal treatment target in patients with ulcerative colitis
Jairath, V.(1,2,3)*;Zou, G.(2,3,4);Wang, Z.(3);Adsul, S.(5);Colombel, J.F.(6);D’Haens, G.R.(7);Freire, M.(5);Moran, G.W.(8,9);Peyrin-Biroulet, L.(10);Sandborn, W.J.(11);Sebastian, S.(12,13);Travis, S.(14);Vermeire , S.(15);Radulescu, G.(3);Sigler, J.(3);Mcfarlane, S.(3);Arya, N.(16);Beaton, M.(1);Bossuyt, P.(17);Green, D.(18);Harlan III, W.(19);Horynski, M.(20);Klopocka, M.(21,22);Petroniene, R.(23);Silverberg, M.S.(24);Wolanski, L.(25);Feagan , B.G.(1,2,3);
(1)University of Western Ontario, Department of Medicine- Division of Gastroenterology, London, Canada;(2)University of Western Ontario, Department of Epidemiology and Biostatistics, London, Canada;(3)Alimentiv Inc., Medical Research and Development, London, Canada;(4)University of Western Ontario, Robarts Research Institute- Schulich School of Medicine and Dentistry, London, Canada;(5)Takeda Pharmaceuticals, Medical, Cambridge, United States;(6)Icahn School of Medicine at Mount Sinai, Division of Gastroenterology, New York, United States;(7)Amsterdam University Medical Centers, Department of Gastroenterology and Hepatology, Amsterdam, The Netherlands;(8)University of Nottingham, Nottingham Digestive Diseases Biomedical Research Centre, Nottingham, United Kingdom;(9)Nottingham University Hospitals NHS Trust, NIHR Nottingham Biomedical Research Centre, Nottingham, United Kingdom;(10)Inserm NGERE U1256- University Hospital of Nancy- University of Lorraine, Department of Gastroenterology, Vandoeuvre-lès-Nancy, France;(11)University of California San Diego, Division of Gastroenterology, La Jolla, United States;(12)Hull Royal Infirmary, Hull and East Yorkshire NHS Trust & Hull and York Medical School, Hull, United Kingdom;(13)Hull York Medical School, School of Health & Life Sciences, Hull, United Kingdom;(14)University of Oxford, Kennedy Institute and Biomedical Research Centre, Oxford, United Kingdom;(15)University Hospitals Leuven- KU Leuven, Department of Gastroenterology and Hepatology, Leuven, Belgium;(16)ABP Research Services Corp., Gastroenterology, Oakville, Canada;(17)Imelda General Hospital, Imelda GI Clinical Research Center, Bonheiden, Belgium;(18)Taunton Surgical Centre, Department of Gastroenterology, Oshawa, Canada;(19)Asheville Gastroenterology Associates, Gastroenterology, Asheville, United States;(20)Endoskopia Sp. z.o.o., Gastroenterology, Sopot, Poland;(21)Collegium Medicum- Nicolaus Copernicus University, Department of Gastroenterology and Nutritional Disorders, Bydgoszcz, Poland;(22)Dr. Jana Biziel University Hospital n 2 in Bydgoszcz, Gastroenterology Clinic, Bydgoszcz, Poland;(23)Barrie GI Associates Inc., Gastroenterology, Barrie, Canada;(24)Toronto Immune and Digestive Health Institute, Gastroenterology, Toronto, Canada;(25)Independent Public Healthcare Center in Łęczna, Gastroenterological Department, Łęcznej, Poland; on behalf of the VERDICT Study Group
Background
The optimal treatment target for ulcerative colitis (UC) is uncertain. The VERDICT trial in moderate to severe UC aims to determine if a treatment target of corticosteroid (CS)-free symptomatic + endoscopic + histologic remission is superior to CS-free symptomatic remission alone. Trial progress and the study cohort to date are described herein.
Methods
Our aim is to enrol 660 patients with moderately to severely active UC (Mayo rectal bleeding subscore [RBS] ≥1; Mayo endoscopic score [MES] ≥2). Patients are randomised to 3 treatment targets (2:3:5 ratio): CS-free symptomatic remission (Mayo RBS = 0) (Group 1); CS-free endoscopic remission (MES ≤1) + symptomatic remission (Group 2); or CS-free histologic remission (Geboes score <2B.0) + endoscopic + symptomatic remission (Group 3). Therapy is administered according to a treatment algorithm that is dependent upon each patient’s UC treatment at screening. Early introduction of vedolizumab (VDZ) and dose escalation up to 300 mg every 4 weeks until the target is met are central to each algorithm. Up to 3 opportunities to achieve the assigned target were prespecified in the algorithms (weeks 16, 32, or 48). Patients and central readers for endoscopy/histopathology are blinded to group allocation, and investigators are unblinded.
Results
As of 2 November 2022, 331 patients were enrolled at 55 sites across 10 North American and European countries. Mean (SD) baseline characteristics (Table) include a UC duration of 8.6 (8.3) years, Mayo Clinic score of 8.7 (1.7), C‑reactive protein level of 13.3 (15.6) mg/dL, and a faecal calprotectin level of 1462.3 (1481.3) mg/kg. At baseline, 52% (172/331) and 11% (37/331) of patients were receiving concomitant CS and immunosuppressives, respectively, and 14% (47/331) had current/prior exposure to tumour necrosis factor antagonists. Most patients (85%) were receiving nonbiologic therapy. Of all randomised patients, 43/69 (Group 1), 65/98 (Group 2), and 115/164 patients (Group 3) have reached week 16; and 28 (65%), 25 (39%), and 42 (37%) met their target, respectively. After a median of 35 weeks (IQR 18-53), a total of 30 (Group 1), 32 (Group 2), and 50 patients (Group 3) have met their target and will be followed to relapse or study termination. The serious adverse event rate (8%; 36/483) was similar across groups.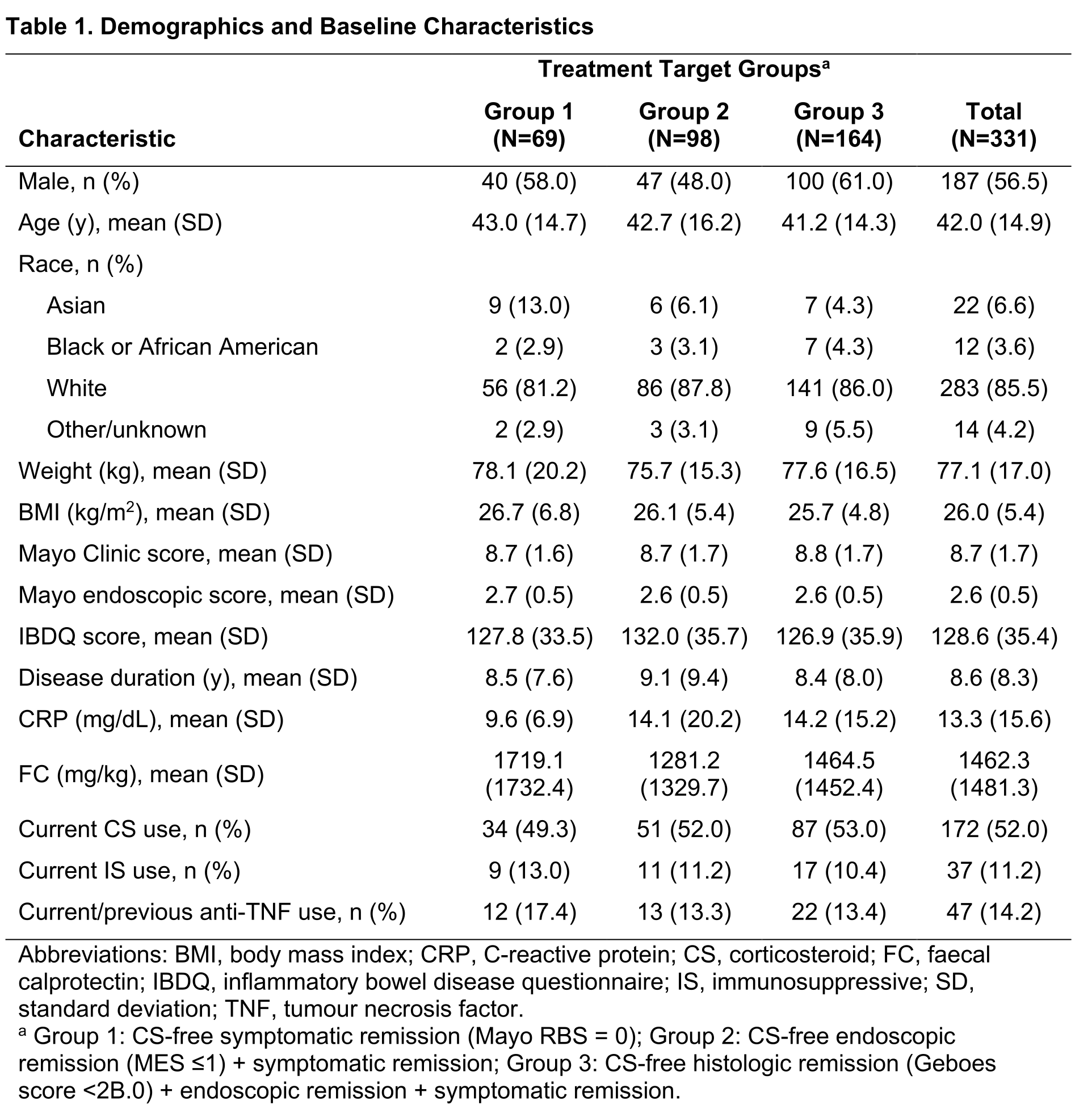
Conclusion
VERDICT has randomised ~50% of its target enrolment. Enrolled patients represent a typical moderate to severe UC population and are primarily bionaive and receiving early VDZ initiation. Early results suggest the feasibility of achieving each treatment target, specifically histologic remission, and are consistent with expected values. No new VDZ safety signals were identified. Completion of enrolment is anticipated in late 2023.


