P589 Effectiveness of partial enteral nutrition to treat adults with Crohn's Disease who lost response to biological therapy
Nardone, O.M.(1)*;Calabrese, G.(2);Alfonsi, L.(3);Rispo, A.(2);La Mantia , A.(2);Fierro, G.(2);Ferrante, M.(2);Testa, A.(2);Guarino, A.D.(2);D'Alessandro, E.(2);Pasanisi, F.(3);Castiglione, F.(2);
(1)University Federico II of Naples, Gastroenterology- Department of Public Health, Naples, Italy;(2)University Federico II of Naples, Gastroenterology- Department of Clinical Medicine and Surgery, Naples, Italy;(3)University Federico II of Naples, Internal Medicine and Clinical Nutrition-Department of Clinical Medicine and Surgery, Naples, Italy;
Background
Partial enteral nutrition (PEN) is a consolidated treatment in children with active Crohn’s disease (CD). However, the benefit of PEN is not well-established for adults with CD. Based on the assumption that diet could aid in treating active disease, we aimed to assess the effectiveness of PEN in combination with biological therapy on transmural response/remission and selected clinical outcomes in adults with CD who lost response to biologics
Methods
We performed a single-centre retrospective observational study by including patients who received PEN due to loss of response to biologics. The primary endpoint was the rate of transmural response/remission at 6 months. We defined transmural remission as a bowel wall thickness ≤3 mm, while transmural response as a decrease in BWT≥25%. Secondary endpoints included clinical remission, defined as Harvey Bradshaw Index<5, and selected clinical outcomes such as surgery, hospitalisations, and change of therapy, including switch and dose/interval escalation at 6 months. Patients were considered adherent when they completed PEN for 6-8 weeks as required for induction of remission, whereas not adherent when they stopped it or if they did not tolerate.
Results
A total of 42 patients,25 males (59,5%) with mean age of 36,1 ±15,6 yo and a mean duration of disease of 138,0 ±113,1 months were enrolled [Table 1]. 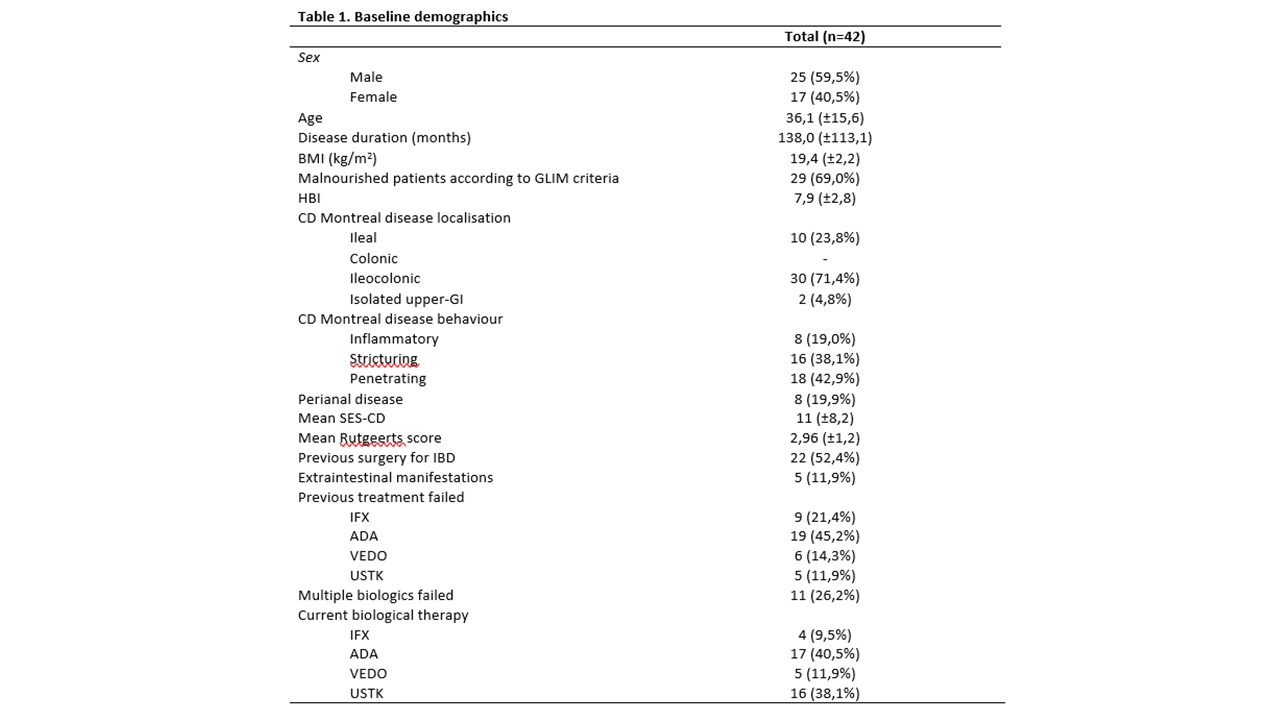
Overall, 14 patients completed PEN at 8 weeks, with a rate of adherence of 33,3%. Whilst 16(38.1%) patients stopped the treatment for intolerance and maintained only biological therapy and 12(28.6%) underwent surgery before 6 months follow-up.At 6 months, patients treated with PEN in combination with biologic had a transmural response of 64,9% compared to 25% treated only with biologics (p=0.03). In both groups, no patients achieved transmural remission. Nevertheless, clinical remission was obtained in 9 (64,3%) patients treated with PEN + biologic compared to 4 (25%) with biologic (p=0.03). Overall,3 patients (18.7%) underwent surgery, all of them were intolerant to PEN. Patients who interrupted PEN and maintained biologics had a higher rate 56,2% of dose escalation/interval and 68,7% changed therapy at six months compared to 7,1% and 14,2% respectively treated with PEN+biologic (p<0.05) [Table 2].At multivariate analysis multiple treatment failures were associated with adherence to PEN (OR=1,583; CI=1,06-2,36; p<0.05).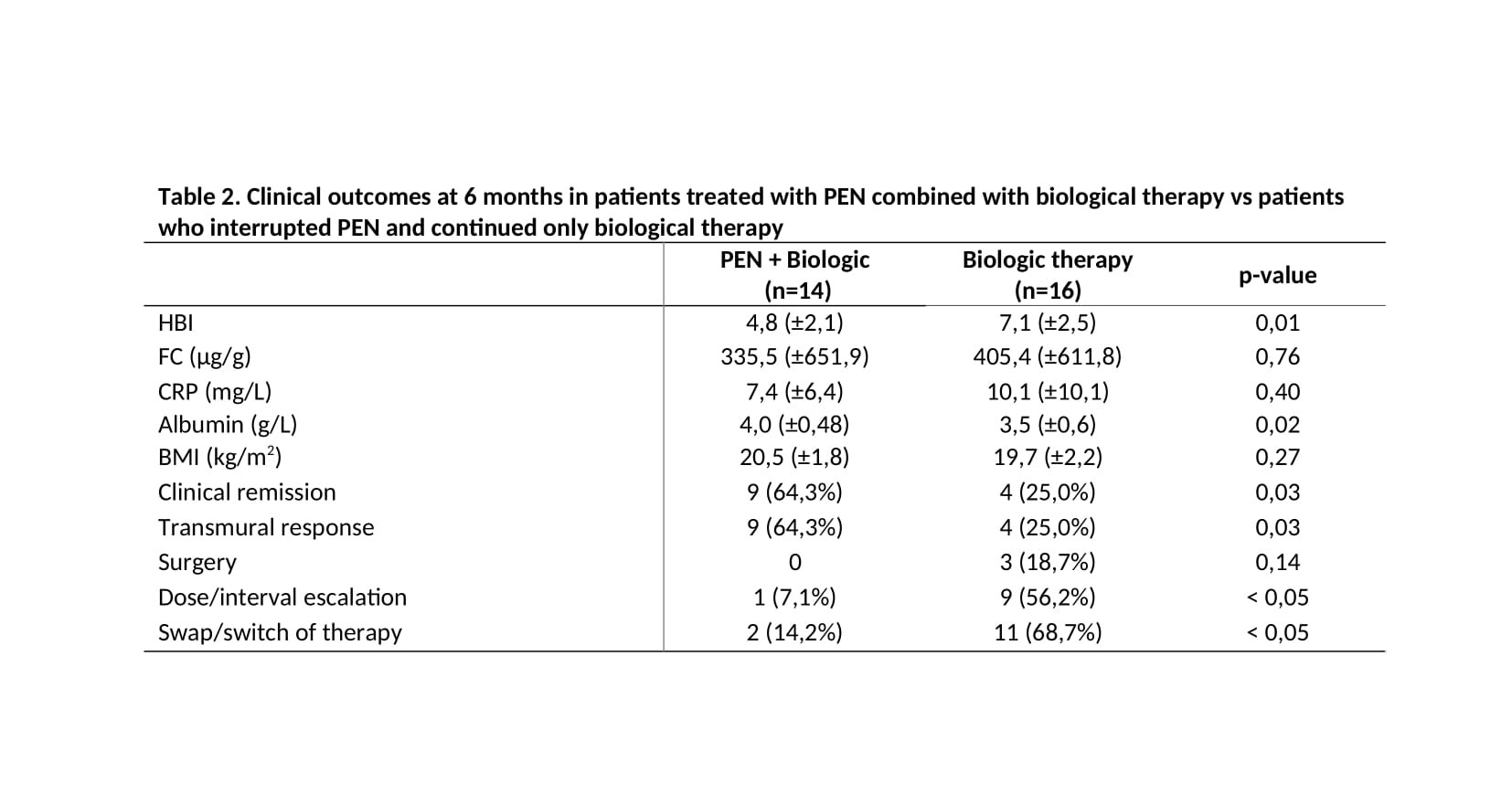
Conclusion
For patients who lost response to biological therapy, the combination with PEN was associated with transmural response and clinical remission. Multiple failures to biologics were associated with adherence to PEN. Hence, the use of PEN dietary should be considered in difficult-to-treat patients


