P591 Biological therapies and small molecules show to be efficacious in patients with moderate-to-severe ulcerative proctitis.
Lemmens, P.(1);Guedelha Sabino, J.(1,2);Dubois, E.(1);Verstockt, B.(1,2);Vermeire, S.(1,2);Ferrante, M.(1,2);
(1)UZ Leuven, Departement of Gastro-enterology and Hepatology, Leuven, Belgium;(2)KU Leuven, Translational Research Centre for Gastrointestinal Disorders TARGID, Leuven, Belgium;
Background
Although adalimumab (ADM), golimumab (GOL), infliximab (IFX), tofacitinib (TFC), ustekinumab (UST) and vedolizumab (VDZ) have been shown efficacious for the treatment of moderate-to-severe ulcerative colitis (UC), data on treatment of patients with ulcerative proctitis (UP, inflammation up to 15cm from the anal verge) are scarce. We therefore aimed to evaluate the effect of these advanced therapies on UP.
Methods
We conducted a retrospective study in which we included 74 patients (median age at induction 41 (IQR 32-52) years) with active UP (Mayo endoscopy sub-score of ≥2) initiating a biological therapy (ADM, GOL, IFX, UST, and VDZ) or small molecule (TFC) in our tertiary referral centre between 02-2005 and 02-2021. The primary endpoint was steroid-free clinical remission at week 20 without prior need for dose optimization. Clinical remission was defined as a total Mayo score ≤2 with no individual sub-score >1. We also evaluated clinical response at short-term follow up (FU), as well as outcomes at long-term FU. The adapted Mayo score (sum of stool frequency subscore and rectal bleeding subscore) was used to rate the long-term treatment outcome, since colonoscopy was not routinely performed. We collected demographic, clinical, biochemical, endoscopic and treatment-related data.
Results
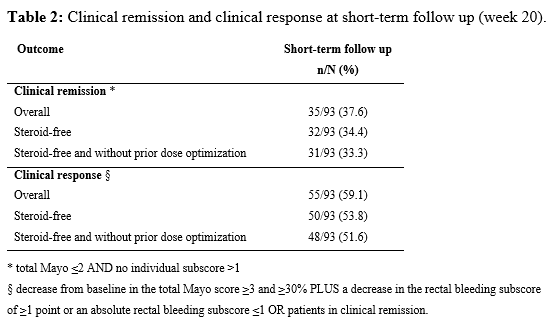
In total 74 patients (57% male) undergoing 93 courses of therapy for UP (16 ADM, 5 GOL, 19 IFX, 4 UST, 44 VDZ, 5 TFC) were included. In Table 1 the baseline characteristics are displayed. At week 20, 31/93 (33.3%) patients achieved steroid-free clinical remission, and 48/93 (51.6%) patients achieved steroid-free clinical response, both without prior need for dose optimization (Table 2). No predictors of steroid-free clinical remission could be identified. Of the 93 patients 91 had a FU under the index-therapy beyond week 20. The median duration of FU was 15.5 (IQR 3-31) months. Of the 31 patients with initial steroid-free clinical remission, 26 patients (83.8%) had sustained steroid-free clinical remission. The treatment-discontinuation-free survival of the entire study population is shown in Figure 1. Only one patient developed a serious adverse event, namely a tuberculosis infection.
Conclusion
Biological therapies and small molecules are a safe treatment for patients with UP, with an overall steroid-free clinical remission rate of 34.4% (32/93) at short-term FU. Our results are lower than in previous studies, probably because of the more stringent definitions of clinical remission and response.