P592 Clinical evaluation of leukocytapheresis compared to vedolizumab in patients with Ulcerative Colitis with contraindications to anti-TNF-alpha: a single-centre experience
Ferronato, A.(1)*;Rigno, M.(2);Rodriguez-Castro, K.I.(1);Brozzi, L.(1);Errigo, G.(2);Castelli, M.(2);Baldassarre, G.(1);
(1)Endoscopy Unit Az. ULSS 7 Pedemontana, Medicine, Santorso VI, Italy;(2)Transfusion Medicine Unit Az. ULSS 8 Berica, Diagnostics, Vicenza, Italy;
Background
Various therapeutic advances have led to a paradigm shift in the clinical management of patients with Inflammatory Bowel Diseases (IBD). Recently vedolizumab and other biologic agents have been introduced as a second choice to anti-TNF-alpha and in whom an anti-TNF-alpha cannot be used. Moreover, leukocytapheresis (LA) is a recognized potential option for the treatment for these IBD patients. The aim of this study is to assess if leukocytapheresis can still have a use in selected Ulcerative Colitis (UC) patients comparing it with vedolizumab.
Methods
From February 2019 to November 2021, 15 patients with UC were evaluated: 8 patients treated with leukocytapheresis (5 weekly apheresis session) (group 1) and 7 with vedolizumab (group 2). All patients had contraindications to anti-TNF-alpha use. The choice of treatment was driven by disease severity, presence of recognized optimal indications for leukocytapheresis and patient’s choice. We evaluated patients by clinical disease activity indexes and blood parameters levels at the beginning of therapy, then after 3 and 12 months.
Results
In group 1 after 12 months of follow up (FU) 5 patients achieved clinical remission with 3 patients receiving monthly maintenance therapy. During the FU 3 patients lost response and started biological therapy (vedolizumab). In group 2 after 12 months 3 patients achieved clinical remission with no treatment failure. In table 1 is shown the clinical disease activity for each patient according to Mayo partial score (MPS). The development of the blood parameters is similar in the two groups, with a general reduction of inflammatory parameters after 12 months. In group 1: leukocyte from 6,1 to 5,8 103/mm3; erythrocyte sedimentation rate (ESR) from 21,1 to 15,6 mm/h; platelets (PLT) from 286,6 to 269,5 103/mm3, faecal calprotectin (FC) from 579,1 to 208,5 mg/kg. In group 2: leukocyte from 8,7 to 7,7; ESR from 42,2 to 45,5; PLT from 320,8 to 266,3; FC from 586,2 to 257,2.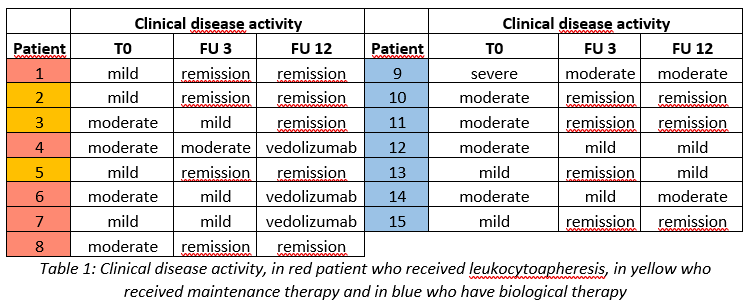
Conclusion
There is an indication overlap between leukocytapheresis and vedolizumab, with a trend in research favouring new biologics. From this clinical experience apheresis appears to be effective as second-choice biological drugs. This type of therapy should not be abandoned but should be investigated further with other comparative study to select patients for the best therapy in terms of efficacy, security and compliance. Moreover, a synergistic approach between these therapies could also be evaluated in the future.


