P595 Validation of a predictive algorithm for thiopurine induced hepatotoxicity in IBD patients
Van Moorsel, F.(1,2);Deben, D.(3);Creemers, R.(4,5);Winkens, B.(6);Pierik, M.(7);Bus, P.(8);Simsek, M.(9);de Boer, N.(9);van Bodegraven, A.(4);Wong, D.(3);
(1)Jeroen Bosch Hospital, Department of Pharmacy, s-Hertogenbosch, The Netherlands;(2)Bernhoven Hospital, Dept. of Clinical Pharmacy, Uden, The Netherlands;(3)Zuyderland Medical Centre, Dept. of Clinical Pharmacy- Clinical pharmacology and Toxicology, Sittard-Geleen/Heerlen, The Netherlands;(4)Zuyderland Medical Centre, Dept. of Gastroenterology- Geriatrics- Internal and Intensive Care Medicine Co-MIK, Sittard-Geleen/Heerlen, The Netherlands;(5)Maastricht University Medical Centre, 5Dev. of Gastroenterology and Hepatology, Maastricht, The Netherlands;(6)Maastricht University Medical Centre, Dept. of methodology and statistics, Maastricht, The Netherlands;(7)Maastricht University Medical Centre, Dev. of Gastroenterology and Hepatology, Maastricht, The Netherlands;(8)Laurentius Hospital, Dept. of Gastroenterology and Hepatology, Roermond, The Netherlands;(9)Amsterdam University Medical Centre, Dept. of Gastroenterology and Hepatology, Amsterdam, The Netherlands;
Background
The thiopurines, azathioprine (AZA) and mercaptopurine (MP), are effective and remain standard treatment options in steroid sparing and maintaining remission in patients with inflammatory bowel disease (IBD). Approximately 25% of patients discontinue within three months after treatment initiation due to adverse events, of which about half due to hepatotoxicity. We hypothesise that identification of patients with an increased risk of adverse events and timely treatment optimisation (e.g. dose adjustment, adjuvant allopurinol) can prevent treatment failure due to adverse events.
The primary objective of this prospective observational multicentre study was to optimise and validate a proposed hepatotoxicity predictive algorithm in IBD patients starting AZA or MP therapy.
Methods
We adapted an algorithm from a previous study to predict the risk of developing hepatotoxicity in thiopurine drug treatment using multivariable logistic regression and a receiver operating characteristic curve. The determinants age, BMI and 6-MMPR-concentration one week after start of treatment (T=1) were inserted as continuous variables in this algorithm. Sex was inserted as a dichotomous variable.
Inclusion criteria were adult thiopurine-naive IBD patients initiating AZA or MP treatment. Subjects were treated according to guidelines and followed for 12 weeks. The primary study outcome was hepatotoxicity within 12 weeks, defined as ALAT > 2x ULN or an R value ((ALAT / ULN ALAT)/(AP / ULN ALP)) ≥ 5. The hepatotoxicity- and no-hepatotoxicity groups were compared using the Mann-Whitney U-test.Results
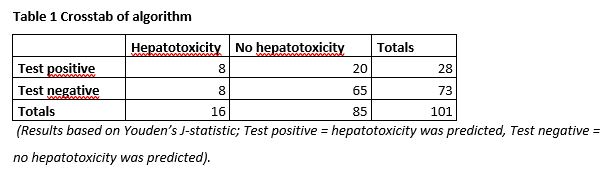
In total, 255 patients were included from Dec 2015 to June 2019, of whom 26 were excluded because of no actual start with AZA or MP (n=18), loss to follow up (n=7) or no IBD diagnosis (n=1). Out of 229 patients 21 (9%) developed hepatotoxicity. Five out of 21 patients (24%) in the hepatotoxicity-group and 123 out of 208 patients (59%) in the no-hepatotoxicity-group had to be excluded for analysis (see Figure 1).
Ninety-three % of patients received MP with a median dose of 0.7 mg/kg (95%CI 0.3-1.4 mg/kg). Median dose of AZA (7%) was 2.0 mg/kg (95%CI 1.1 - 2.6 mg/kg).
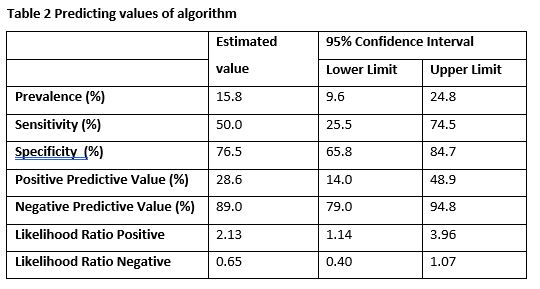
There was a difference between the hepatotoxicity and no-hepatotoxicity group in BMI (28 versus 24, p=0.022) and 6-MMPR/6-TGN ratio at T=1 (16 versus 9, p=0.027). In Table 1, the number of true positives, true negatives, false positives and false negatives are presented. The diagnostic values are shown in Table 2. A specificity of 77% (95%CI 66-85%) and a sensitivity of 50% (95%CI 26-75%) was obtained.
Conclusion
In conclusion, the adapted algorithm does not accurately predict hepatotoxicity in this cohort of patients on relatively low-dose thiopurine treatment.