P598 Indicators for inadequate response among patients with Ulcerative Colitis treated with advanced therapies in German clinical practice
Bokemeyer, B.(1);Picker, N.(2);Kromer, D.(2);Rosin, L.(3);Patel, H.(4);
(1)Gastroenterology Practice, Minden, Minden, Germany;(2)Ingress Health HWM, GmbH, Wismar, Germany;(3)Galapagos Biopharma Deutschland, GmbH, München, Germany;(4)Galapagos, Nv, Mechelen, Belgium;
Background
Management of Ulcerative Colitis (UC) is challenging, and clinicians are often obliged to attempt a variety of therapies in sequence until an adequate clinical response is achieved. This study examined the rates of inadequate response to advanced therapies among UC patients in clinical practice in Germany.
Methods
Using a retrospective chart review, patients with UC treated with an advanced therapy (adalimumab, infliximab, golimumab, vedolizumab, and tofacitinib) between 01/2017 through 09/2019 were selected from 18 outpatient gastroenterology practices across Germany. Patients with at least 12 months of data before (baseline) and after initiating an advanced therapy (Index Date) were included. Data was collected up to 24 months after index date. Inadequate response was defined as at least one of the following events: index therapy discontinuation due to lack of response, therapy escalation, augmentation with newly prescribed conventional therapies, corticosteroid (CS) dependency (use for ≥12 weeks), CS use during the maintenance phase, UC-related hospitalisations, surgery or emergency visit. Subgroups included patients with prior exposure to biologics, with and without remission (clinical remission defined as partial Mayo Score ≤1) at one year, and concurrent use of CS at the index date. Kaplan-Meier analysis was used to examine the rates over time. Patients were censored at the end of the study period.
Results
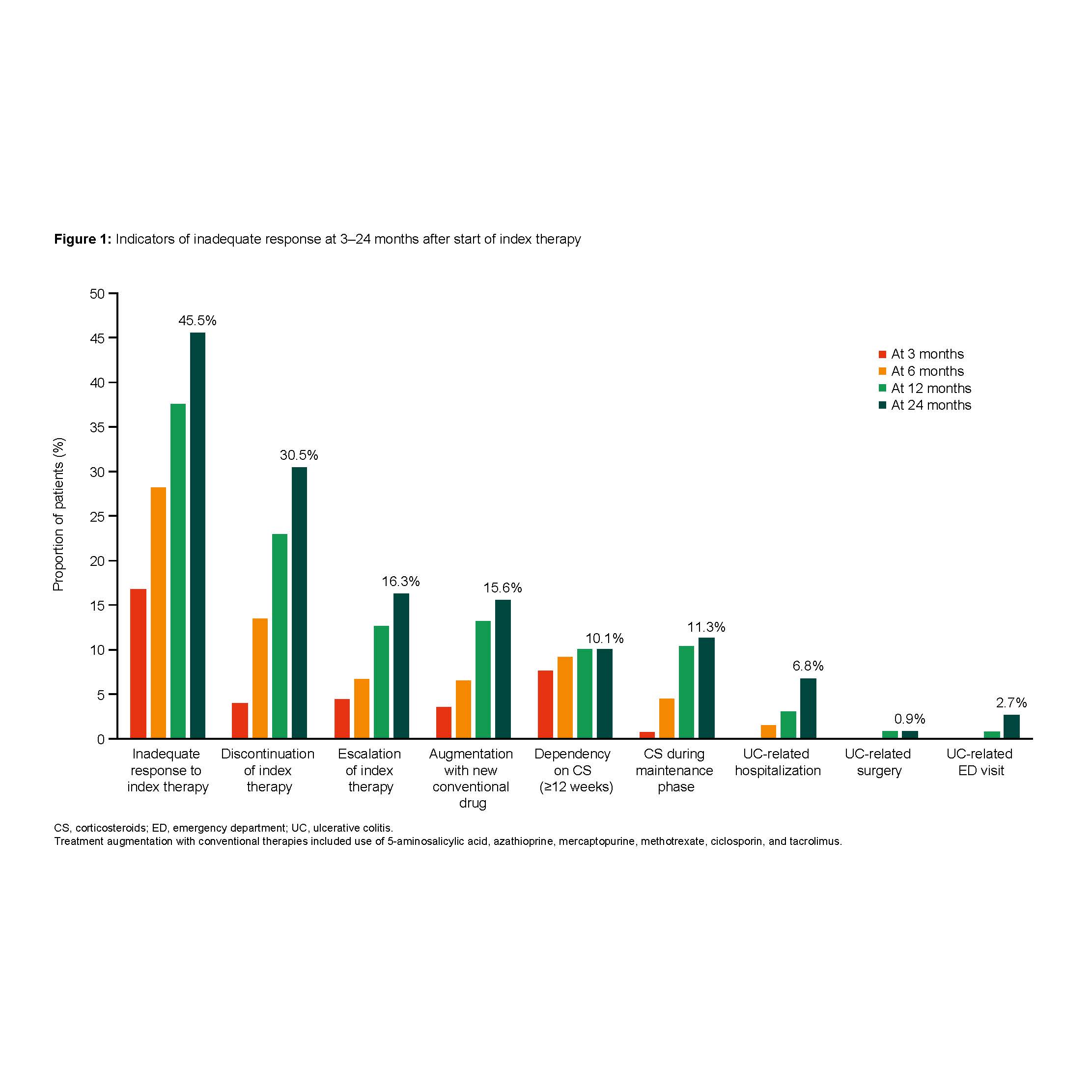
Among 149 patients (females: 50.3%; median age: 40 years; median follow-up: 25.9 months), 96 (64.4%) patients were biologic-naïve. Nearly half of the patients (45.5%) had ≥1 indicator for an inadequate response within two years after the index date (Figure 1). Among those who discontinued (N=43), 82.0% had switched to another advanced therapy. Therapy escalation was observed in 20 (16.3%) patients, and augmentation with conventional therapies was observed in 19 (15.6%) patients. 14 patients (11.3%) received CS during the maintenance phase of the index therapy, and 14 patients (10.1%) were CS dependent for ≥12 weeks. Inadequate response rates were significantly higher in patients without (n=80) vs. with remission (n=54) after one year (53.4% vs. 35.5%; p<0.05, Figure 2) and in patients with (n=42) vs. without concurrent CS (n=107) at the index date (67.0% vs. 36.9%; p<0.05). There was no significant difference between biologic-naïve and biologic-experienced patients.

Conclusion
Nearly half of the patients with UC experienced an inadequate response to their advanced therapy within two years. Higher rates of inadequate response were observed in patients without remission and in those with concurrent use of CS at baseline. More effective therapies are needed to achieve better outcomes in UC.


