P603 Real-world Scottish experience of anti-TNF therapy in paediatric Crohn’s disease 2016-2020 against the ECCO-ESPGHAN recommendations
Wands , D.(1)*;Scott , G.(2);Wilson , D.C.(1);Hansen , R.(2);Chalmers , I.(3);
(1)Royal Hospital for Children and Young People, Department of Paediatric Gastroenterology, Edinburgh, United Kingdom;(2)Royal Hospital for Children, Department of Paediatric Gastroenterology, Glasgow, United Kingdom;(3)Royal Aberdeen Children’s Hospital, Department of Paediatric Gastroenterology, Aberdeen, United Kingdom;
Background
ECCO-ESPGHAN updated the guideline on the management of paediatric Crohn’s disease (CD) in 2021. This promoted a move to early risk stratification and a ‘top-down’ (anti-TNF within 4 weeks of diagnosis) approach for patients who were deemed high-risk. High-risk was defined by extensive panenteric or severe disease, perianal, stricturing and/or penetrating behaviour. We sought to compare the use of anti-TNF therapy against the new guidance by conducting a Scottish nationwide retrospective multi-centre study (all three regional PGHAN units).
Methods
We analysed a prospectively identified nationwide cohort of all new paediatric CD patients (<18 years) diagnosed in Scotland between 01/01/16 and 31/12/20. We retrospectively collected data from electronic medical records on the use of anti-TNF therapy (infliximab or adalimumab) within 18 months of diagnosis. Paris location and behaviour at diagnosis were determined, allowing us to split the patient group into high-risk or low/medium-risk as per latest ECCO-ESPGHAN guidance. Data regarding surgical intervention was collected and defined as: Perianal (Seton suture or drainage of abscess) and/or bowel resection +/- stoma formation.
Results
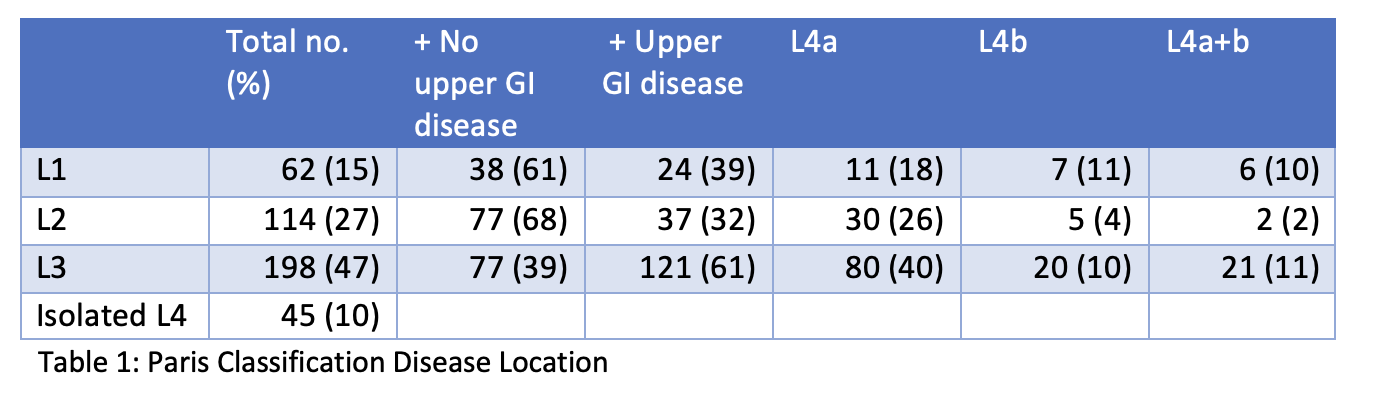
419 patients were included (259/419 male; median age at diagnosis 13.2 yrs). Disease phenotype and behaviour are shown in tables 1 and 2. 225/419 (54%) were classified as high-risk and 194/419 (46%) as low/medium-risk. 171/225 (76%) high-risk and 78/194 (40%) low/medium-risk patients received anti-TNF within 18 months of diagnosis, with median start at 5 months (IQR: 1- 8 m) and 6.5 months (IQR: 3 – 13 m) respectively. Figure 1 demonstrates cumulative anti-TNF new starts by month. Of these patients, 49/171 (40%) high-risk and 12/78 (15.3%) low-risk received anti-TNF within 4 weeks of diagnosis. High-risk patients were more likely to receive anti-TNF (76.0% vs 40.2%, p<0.0001). 37 (8.8%) patients underwent surgical intervention; 31/225 (13.7%) high-risk and 6/194 (3.1%) low/medium-risk (13.7% vs 3.1%, p= 0.0002). 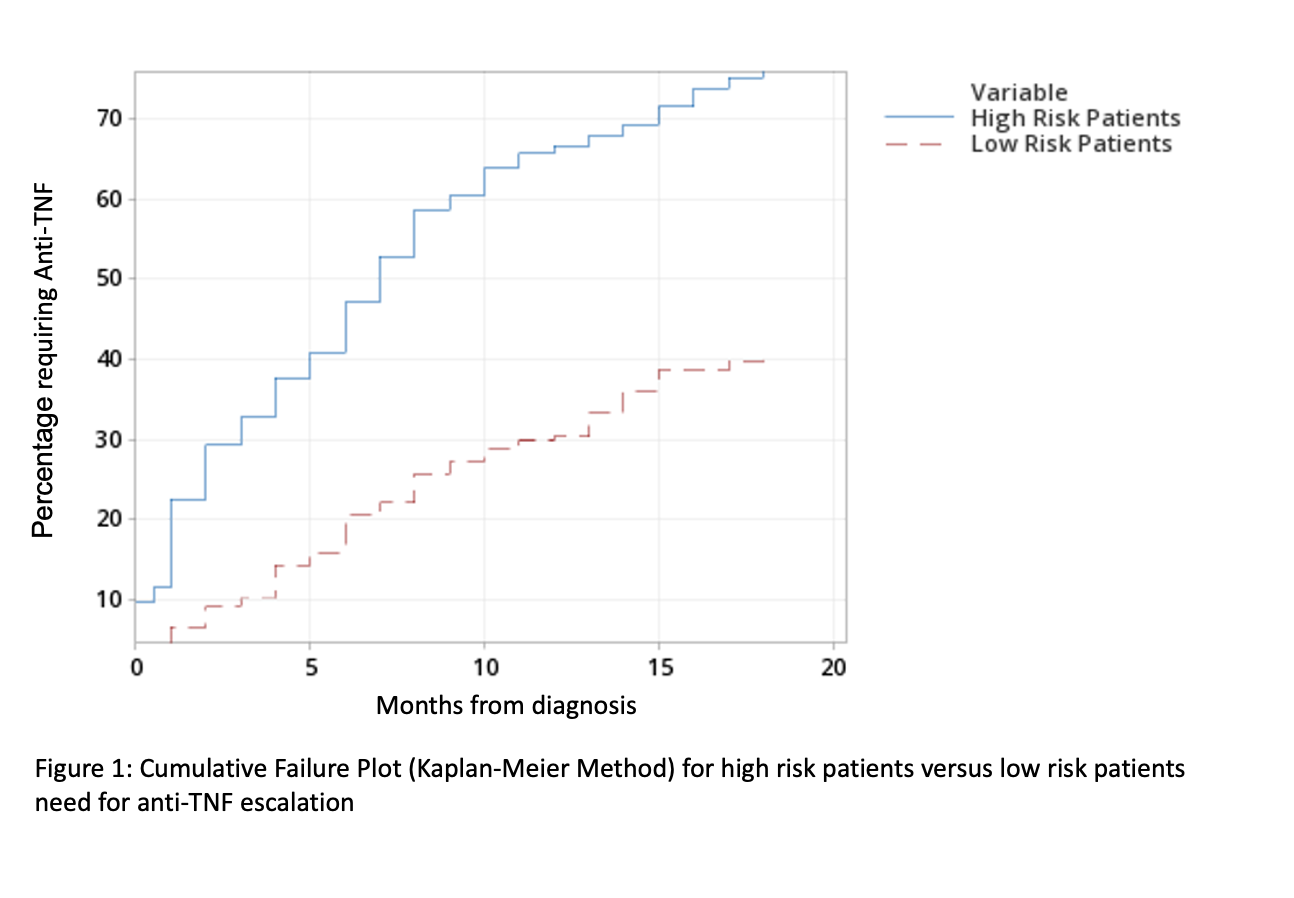

Conclusion
The ECCO-ESPGHAN guidance seeks to shift practice towards “top-down” anti-TNF therapy in high-risk patients. Our real-world data supports this view by showing that high-risk patients are more likely to require biologics within 18 months and more likely to require surgery than low/medium-risk patients. The ECCO-ESPGHAN approach would however have led to unnecessary anti-TNF in 24% of our high-risk cohort within 18 months of diagnosis. The financial cost and risk:benefit profile of this group should be considered carefully both in clinical practice and future guidance.


