P607 Efficacy and safety of tofacitinib in Ulcerative Colitis patients with extraintestinal manifestations in OCTAVE Open
Rubin , D.T.(1);Vavricka , S.R.(2);Armuzzi , A.(3);Dubinsky , M.C.(4);Sharara , A.I.(5);Modesto , I.(6);Fellmann , M.(7);Liguori , G.(8);Lawendy , N.(9);Cadatal , M.J.(10);Lichtenstein , G.R.(11);
(1)-, University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago- IL, United States;(2)University Hospital Zürich, Department of Gastroenterology and Hepatology, Zürich, Switzerland;(3)Fondazione Policlinico Universitario A. Gemelli IRCCS – Università Cattolica del Sacro Cuore, IBD Unit, Rome, Italy;(4)-, Icahn School of Medicine at Mount Sinai, New York- NY, United States;(5)American University of Beirut Medical Center, Division of Gastroenterology, Beirut, Lebanon;(6)-, Pfizer Inc, New York- NY, United States;(7)-, Pfizer Switzerland AG, Zürich, Switzerland;(8)-, Pfizer Srl, Rome, Italy;(9)-, Pfizer Inc, Collegeville- PA, United States;(10)-, Pfizer Inc, Manila, Philippines;(11)Perelman School of Medicine at the University of Pennsylvania, Division of Gastroenterology, Philadelphia- PA, United States;
Background
Tofacitinib is an oral, small molecule JAK inhibitor for the treatment of ulcerative colitis (UC). Data from OCTAVE Induction 1&2 and OCTAVE Sustain demonstrated that a history of extraintestinal manifestations (EIMs) was shown not to influence the efficacy of tofacitinib 10 mg twice daily (BID).1 We explored the efficacy and safety of tofacitinib in patients (pts) with and without a history of EIMs in the open-label, long-term extension study, OCTAVE Open.
Methods
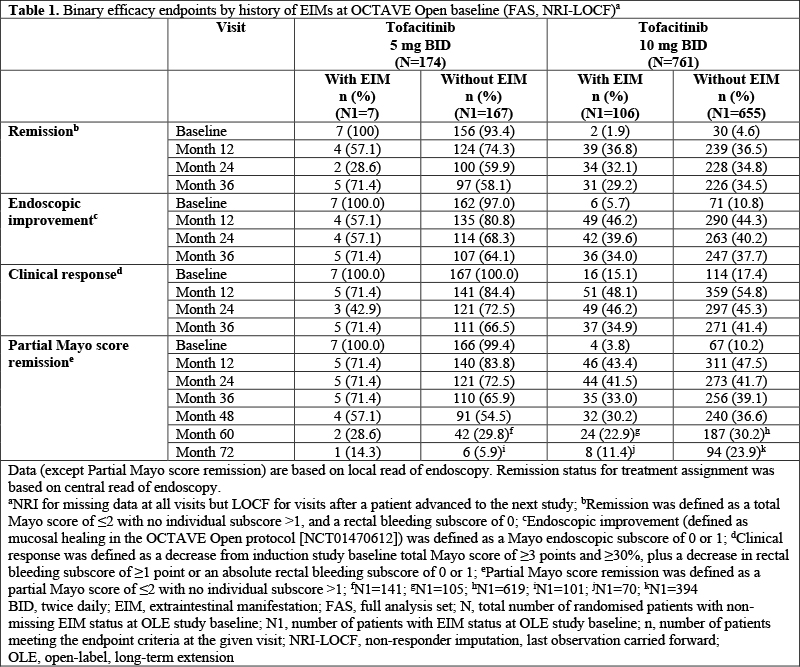
Efficacy and safety (treatment-emergent serious adverse events of interest) data from OCTAVE Open (NCT01470612) were analysed by history of EIMs. Tofacitinib dose in OCTAVE Open was based on baseline remission status; pts in remission received tofacitinib 5 mg BID; all others received 10 mg BID. The frequency of pre-defined prior and active EIMs at OCTAVE Open baseline (peripheral arthritis [PA]; sacroiliitis; ankylosing spondylitis [AS]; myopathy; pyoderma gangrenosum; erythema nodosum; scleritis, episcleritis; uveitis, iritis; oral ulcer/stomatitis; and thromboembolic disorder) and new occurrences of EIMs at Month (M)36, or at early termination, were evaluated in pts with non-missing data.
Results
In OCTAVE Open, 113/935 (12.1%) had EIMs at baseline (7/174 [4.0%] tofacitinib 5 mg BID; 106/761 [13.9%] tofacitinib 10 mg BID). Pts with EIMs were more likely to be female (61.1%), have a total Mayo score of ≥3 (93.8%) at OCTAVE Open baseline and have disease duration ≥6 years (60.2%), prior tumour necrosis factor inhibitor failure (61.9%), prior immunosuppressant treatment (86.7%) or corticosteroid use (54.0%) at induction study baseline, compared to those without EIMs (38.8%, 78.7%, 49.6%, 50.9%, 74.5% and 43.9%, respectively). There was no consistent trend in the proportions of pts with and without EIMs at baseline who met the OCTAVE Open efficacy endpoints (Table 1). Tofacitinib 5 and 10 mg BID had comparable safety in pts with or without EIMs at baseline (Table 2). In pts with EIMs at baseline, the most frequent active EIMs were PA (51/113 [45.1%]), sacroiliitis (6/113 [5.3%]), oral ulcer/stomatitis (5/113 [4.4%]), myopathy (4/113 [3.5%]) and AS (4/113 [3.5%]). At M36, 4/428 [0.9%] had new occurrences of PA and there were no new occurrences of sacroiliitis (0/468 [0%]), oral ulcer/stomatitis (0/460 [0%]), myopathy (0/469 [0%]) or AS (0/469 [0%]).
Conclusion
The presence or absence of a history of EIMs at baseline did not impact the long-term efficacy and safety of tofacitinib in pts with UC. New occurrences of EIMs were infrequent.
Reference:
1. Rubin DT et al. Therap Adv Gastroenterol 2021; 14: 1-12.